Patients with semantic dementia (SD), a neurodegenerative disease affecting the anterior temporal lobes (ATL) (1), present with striking cognitive deficits: they can have difficulties naming objects and familiar people from both pictures and descriptions (2, 3). Furthermore, SD patients make semantic errors (e.g., naming “horse” a picture of a zebra), suggesting that their impairment affects object knowledge rather than lexical retrieval. Because SD can affect object categories as disparate as artifacts, animals, and people, as well as multiple input modalities, it has been hypothesized that ATL is a semantic hub (4) that integrates information across multiple modality-specific brain regions into multimodal representations. With a series of converging experiments using multiple analysis techniques, Wang et al. (5) test the proposal that ATL is a semantic hub in the case of person knowledge, investigating whether ATL: (i) encodes multimodal representations of identity, and (ii) mediates the retrieval of knowledge about people from representations of perceptual cues.
Wang et al. (5) asked 50 participants to learn biographical information (name, age, marital status, occupation, city of residence, and the appearance of their house as presented in a photograph) about four fictitious people over 2 d of training. On the third day, participants completed an fMRI experiment in which they were shown images of the four people’s faces (pictured from different viewpoints with respect to the training), their names (written in a different font and color), their houses (pictured from different viewpoints), and images of objects associated with their occupation. Multimodal representations of person identity were probed in a set of regions-of-interest (ROIs), training a support vector machine to classify between different face identities, and testing it to classify between the associated names, houses, and occupation-related objects. All four classifications were significant in the ATL, and none of the classifications was significant in any of the other ROIs, showing that ATL representations of person identity generalize across a variety of stimulus types. The ATL has been previously shown to encode information about face identity (6), generalizing across facial expressions (7), viewpoints (8), and face parts (9). The ATL has also been implicated in the association of names and faces with knowledge about people (10, 11). Wang et al.’s (5) classification results are an important extension of this work, revealing representations of identity that generalize across faces, names, and objects associated with one individual.
In the ATL-hub hypothesis, the ATL acts as an intermediary for the linkage of different kinds of knowledge represented in specialized brain regions. To test this proposal, in a second study, Wang et al. (5) defined a set of ROIs showing stronger responses when retrieving information about a person compared with a resting baseline. The contrast identified as ROIs the left ATL, hippocampus, posterior cingulate (PCC), inferior parietal lobule (IPL), fusiform face area (FFA), and visual word form area (VWFA). Dynamic causal modeling was used to assess three alternative models of task-dependent modulation of effective connectivity between the ROIs. A model in which retrieval of person knowledge represented in the IPL and PCC from perceptual cues was mediated by the ATL (“ATL as hub”) was selected as optimal by Bayesian model selection, outperforming a model in which retrieval was achieved directly from representations of the perceptual cues in the FFA/WVFA, and a model in which retrieval was mediated by the hippocampus. Furthermore, using psychophysiological interactions, Wang et al. (5) showed that the ATL increased its connectivity with the IPL during the retrieval of a person’s status, but with the PCC during the retrieval of a person’s personality traits. Multivariate pattern analyses showed that status could be decoded from the IPL (and from none of the other ROIs), and personality traits could be decoded from the PCC (and from none of the other ROIs). These connectivity results, considered in isolation, are also consistent with the ATL being an intermediate perceptual processing step between upstream perceptual representations in the FFA/VWFA and semantic representations in the IPL and PCC (Fig. 1). However, the classification of identity across different modalities obtained in the first study argues against this possibility. Further strengthening the semantic hypothesis, a recent study showed that the ATL encodes knowledge about where objects are typically found (e.g., the kitchen or the garage), and about the actions performed to use them (12).
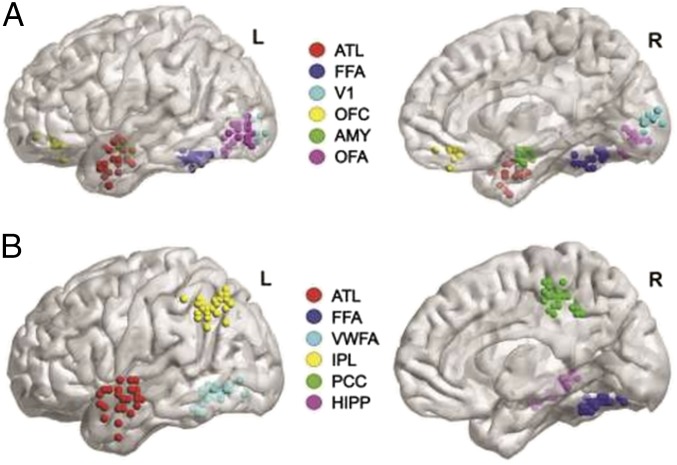
Fig. 1.
(A) ROI for study 1 in Wang et al. (5). (B) ROI for study 2 in Wang et al. (5). AMY, amygdala; HIPP, hippocampus; ITG, inferior temporal gyrus; OFA, occipital face area; OFC, orbitofrontal cortex face patch; STG, superior temporal gyrus; STS, superior temporal sulcus.
If the ATL is a semantic hub, how is it organized? The Wang et al. (5) results converge with the previous literature in suggesting that subregions of the ATL are specialized for different object domains. In addition to regions showing differential responses to faces (13), person knowledge (14), and theory of mind (15), subregions of the ATL respond differentially to animals over artifacts and to artifacts over animals (16). These regions correspond closely to regions whose damage is associated with deficits for the recognition of those categories of objects (17, 18).
Variants of SD that disproportionately affect the left ATL lead to greater deficits for recognizing people from their name, whereas variants of SD that disproportionately affect the right ATL lead to greater deficits for recognizing people from their faces (19). Ventral regions of the ATL show stronger responses to pictures, whereas dorsal regions show stronger responses to auditory words (20, 21).
Taken together, these results suggest that although subregions of the ATL encode multimodal representations (5), both stimulus modality and stimulus domain are principles driving the large-scale topographic organization of the ATL. A systematic and comprehensive investigation within individual participants of ATL responses to stimuli in different domains and modalities is still lacking in the literature. Another key result would be to show that multimodal classification and hub-like connectivity occur in the same ROI: in the two studies by Wang et al. (5), the ATL ROIs were defined with different contrasts (Fig. 1).
The study by Wang et al. (5) lends strong support to the hypothesis that the ATL is a hub interfacing perceptual representations with stored knowledge. Should we conclude that the ATL is the “seat of semantics”? A meta-analysis of 120 neuroimaging studies using semantic contrasts (words > nonwords, semantic > phonological tasks) identified a set of regions including the ATL, as well as the angular gyrus, PCC, and medial prefrontal cortex (22). Both the PCC and the angular gyrus have been recently shown to encode multimodal representations of people and places (23, 24). Furthermore, a recent study (25) has implicated the angular gyrus in compositional semantics, showing that this region is differentially activated by meaningful over nonmeaningful word combinations, and that in neuropsychological patients the amount of atrophy in the angular gyrus correlates with the amount of impairment for the processing of combinatorial concepts.
These lines of evidence suggest that there may not be a single “seat of semantics” in the human brain, but point to a crucial role of the ATL in the semantic system. The approach of using converging analysis techniques across multiple studies adopted by Wang et al. (5) holds promise to deepen our understanding not only of the ATL, but also of the other regions contributing to the representation and processing of semantic knowledge.
Footnotes
The author declares no conflict of interest.
See companion article on page E3305.
References
- 1.Rosen HJ, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- 2.Warrington EK. The selective impairment of semantic memory. Q J Exp Psychol. 1975;27:635–657. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- 3.Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- 4.Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, et al. Dynamic neural architecture for social knowledge retrieval. Proc Natl Acad Sci USA. 2017;114:E3305–E3314. doi: 10.1073/pnas.1621234114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kriegeskorte N, Formisano E, Sorger B, Goebel R. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proc Natl Acad Sci USA. 2007;104:20600–20605. doi: 10.1073/pnas.0705654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nestor A, Plaut DC, Behrmann M. Unraveling the distributed neural code of facial identity through spatiotemporal pattern analysis. Proc Natl Acad Sci USA. 2011;108:9998–10003. doi: 10.1073/pnas.1102433108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anzellotti S, Fairhall SL, Caramazza A. Decoding representations of face identity that are tolerant to rotation. Cereb Cortex. 2014;24:1988–1995. doi: 10.1093/cercor/bht046. [DOI] [PubMed] [Google Scholar]
- 9.Anzellotti S, Caramazza A. From parts to identity: Invariance and sensitivity of face representations to different face halves. Cereb Cortex. 2016;26:1900–1909. doi: 10.1093/cercor/bhu337. [DOI] [PubMed] [Google Scholar]
- 10.Tsukiura T, Suzuki C, Shigemune Y, Mochizuki-Kawai H. Differential contributions of the anterior temporal and medial temporal lobe to the retrieval of memory for person identity information. Hum Brain Mapp. 2008;29:1343–1354. doi: 10.1002/hbm.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsukiura T, et al. Dissociable roles of the anterior temporal regions in successful encoding of memory for person identity information. J Cogn Neurosci. 2010;22:2226–2237. doi: 10.1162/jocn.2009.21349. [DOI] [PubMed] [Google Scholar]
- 12.Peelen MV, Caramazza A. Conceptual object representations in human anterior temporal cortex. J Neurosci. 2012;32:15728–15736. doi: 10.1523/JNEUROSCI.1953-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajimehr R, Young JC, Tootell RB. An anterior temporal face patch in human cortex, predicted by macaque maps. Proc Natl Acad Sci USA. 2009;106:1995–2000. doi: 10.1073/pnas.0807304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zahn R, et al. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci USA. 2007;104:6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodell-Feder D, Koster-Hale J, Bedny M, Saxe R. fMRI item analysis in a theory of mind task. Neuroimage. 2011;55:705–712. doi: 10.1016/j.neuroimage.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 16.Anzellotti S, Mahon BZ, Schwarzbach J, Caramazza A. Differential activity for animals and manipulable objects in the anterior temporal lobes. J Cogn Neurosci. 2011;23:2059–2067. doi: 10.1162/jocn.2010.21567. [DOI] [PubMed] [Google Scholar]
- 17.Brambati SM, et al. The anatomy of category-specific object naming in neurodegenerative diseases. J Cogn Neurosci. 2006;18:1644–1653. doi: 10.1162/jocn.2006.18.10.1644. [DOI] [PubMed] [Google Scholar]
- 18.Bi Y, et al. The role of the left anterior temporal lobe in language processing revisited: Evidence from an individual with ATL resection. Cortex. 2011;47:575–587. doi: 10.1016/j.cortex.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Snowden JS, Thompson JC, Neary D. Knowledge of famous faces and names in semantic dementia. Brain. 2004;127:860–872. doi: 10.1093/brain/awh099. [DOI] [PubMed] [Google Scholar]
- 20.Anzellotti S, Caramazza A. Multimodal representations of person identity individuated with fMRI. Cortex. 2017;89:85–97. doi: 10.1016/j.cortex.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Visser M, Jefferies E, Lambon Ralph MA. Semantic processing in the anterior temporal lobes: A meta-analysis of the functional neuroimaging literature. J Cogn Neurosci. 2010;22:1083–1094. doi: 10.1162/jocn.2009.21309. [DOI] [PubMed] [Google Scholar]
- 22.Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fairhall SL, Caramazza A. Brain regions that represent amodal conceptual knowledge. J Neurosci. 2013;33:10552–10558. doi: 10.1523/JNEUROSCI.0051-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fairhall SL, Anzellotti S, Ubaldi S, Caramazza A. Person- and place-selective neural substrates for entity-specific semantic access. Cereb Cortex. 2014;24:1687–1696. doi: 10.1093/cercor/bht039. [DOI] [PubMed] [Google Scholar]
- 25.Price AR, Bonner MF, Peelle JE, Grossman M. Converging evidence for the neuroanatomic basis of combinatorial semantics in the angular gyrus. J Neurosci. 2015;35:3276–3284. doi: 10.1523/JNEUROSCI.3446-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]