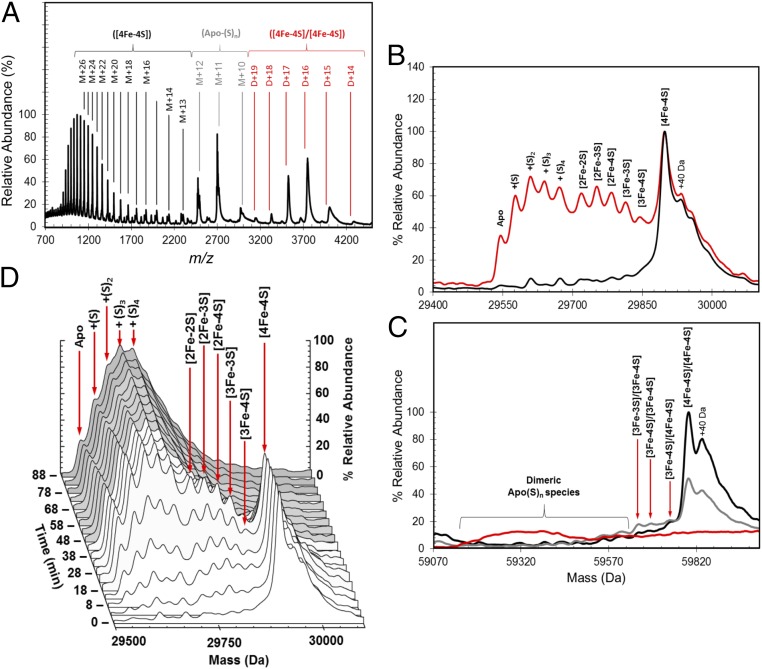
Fig. 2.
ESI-MS of [4Fe-4S] FNR and the effect of exposure to O2. (A) Full m/z spectrum for FNR. Charge states corresponding to monomeric [4Fe-4S] (black), persulfide adducts of apo (gray), and dimeric [4Fe-4S] (red) FNR species are shown. (B) Deconvoluted mass spectrum of [4Fe-4S] S24F FNR (black line) before and after exposure to dissolved atmospheric oxygen (18 min exposure; red line). This exposure results in the formation of a variety of protein-bound clusters, including [3Fe-4S], [3Fe-3S], and [2Fe-2S] forms. Persulfide adducts of [2Fe-2S] and apoprotein are also observed. (C) Deconvoluted spectrum of dimeric FNR showing the presence of the [4Fe-4S]/[4Fe-4S] form under anaerobic conditions and only low-intensity features caused by cluster conversion species after the addition of O2 [20 (gray line) and 80 min (red line)]. The longer exposure time resulted in the loss of all cluster-containing dimer species, and the appearance of a broad, poorly resolved feature centered on 59,320 Da, approximating the mass of FNR containing multiple sulfane sulfurs (S0n, nave ∼ 8). (D) 3D plot showing the formation and decay of all monomeric FNR species during the O2 reaction time course. Spectra are representative of multiple repeat experiments.