Abstract
Obstructive sleep apnea syndrome (OSAS) is the most common type of sleep disorder which is associated with a series of cardiovascular disorders, including right ventricular (RV) dysfunction. However, it is difficult to assess the RV function systematically using a conventional echocardiography because RV has a complex geometrical shape. A case-control study was performed to assess the regional right ventricular potential dysfunction in patients with OSAS using velocity vector imaging (VVI) from March 2014 to October 2015.
Sixty-nine patients with OSAS were divided into 3 groups: mild, moderate, and severe according to the apnea–hypopnea index (AHI). A total of 31 cases of healthy subjects were enrolled as the control group. Digital images of apex 4-chamber views were acquired to measure the peak systolic velocity (V), strain (ST), and strain rate (STR) of right ventricular free wall (RVFW) basal, middle, and apical segments using VVI.
The peak systolic velocity of RVFW basal segments in the mild OSAS group increased (t = 2.22, P = 0.049) and gradually reduced in the moderate and severe groups compared with the controls. The values of systolic ST and STR of apical segments decreased in the mild OSAS group compared with the normal control group (t = 3.30, P = 0.02; t = 3.75, P = 0.01, respectively), and decreased furthermore in the moderate and severe OSAS groups.
The change in the right ventricular regional systolic function starts before the development of heart dysfunction and pulmonary hypertension. At the early stage of OSAS, the deformation decreases in the RVFW apical segment, and the peak systolic velocities increase in the RVFW basal segment. The VVI is a sensitive method which is expected to be a worthy technique for early clinical therapy in patients with OSAS.
Keywords: obstructive sleep apnea syndrome, right ventricular function, velocity vector imaging
1. Introduction
Obstructive sleep apnea syndrome (OSAS) is the most common type of sleep disorder caused by repetitive partial or complete upper airway collapse during sleep.[1] It occurs more than 5 times per hour during sleep.[2] Patients with OSAS always complain of snoring, sleep fragmentation, waking up with a choking sensation, excessive sleepiness, unrefreshing sleep, fatigue or tiredness, and morning headache. OSAS is considered as a global health problem because of its negative effect on the quality of life and high prevalence.[3] OSAS leads to systemic hypertension[4] and associates with a variety of cardiovascular disorders, including right ventricular (RV) dysfunction, arrhythmia, hypertrophy, coronary artery disease, and heart failure.[5–8]
Episodes of obstructive hypopneas or apneas result in intermittent hypoxia, autonomic nervous dysfunction, and consequently may have a direct or an indirect adverse effect on RV myocardial function even in patients without cardiac or obstructive pulmonary diseases.[9,10] The right ventricle plays an important role in the morbidity and mortality of patients presenting with signs and symptoms of cardiopulmonary disease.[11,12] However, the right ventricle has a more complex geometrical shape and is wrapped around the left ventricle.[13] Therefore, it is difficult to assess the RV function systematically using a conventional 2-dimensional (2D) echocardiography.[14] The tissue Doppler echocardiography method is also used for evaluating RV dysfunction, but it has high interobservational variability and is susceptible to the cardiac translational motion.[15,16] The 2D speckle tracking echocardiography (2D-STE) method is a more sensitive method for assessing myocardial deformation characteristics. However, Park et al[17] showed that the 2D-STE method underestimates the longitudinal strain of septum and global RV strain when compared with cardiac magnetic resonance, which is a “gold standard” in patients with ischemic heart disease.
The velocity vector imaging (VVI) method, without the limitation of a Doppler beam angle, is a unique technology that assesses myocardial movement and deformation by detecting and tracking speckles in the myocardium on grayscale (B-mode) images. The offline analysis of VVI displays several parameters, including tissue velocities (V), strain (ST), and strain rate (STR), to quantify global and regional ventricular functions. The purpose of this study was to investigate the regional RV systolic function in patients with OSAS and evaluate the application of VVI technology.
2. Methods
2.1. Determination of patients and control groups
From March 2014 to October 2015, 69 patients (42 men, 27 women; age, 30–60 years) diagnosed with OSAS for the first time at the University Affiliated Hospital (Qingdao, China) were included in this study after conducting overnight polysomnographies at the sleep laboratory. To create a control group, 31 age- and sex-matched healthy individuals (19 men, 12 women; age, 30–60 years) were chosen after obtaining detailed history and performing physical examination, routine blood examination, electrocardiogram, overnight polysomnography, and transthoracic echocardiography, including Doppler and ST studies. Written informed consent was obtained from all patients included in this study, which was approved by the local ethics committee of the Qingdao University Medical School, Qingdao, China.
Hypopnea was defined as 30% or more reduction in the respiratory airflow lasting for >10 s and accompanied by a decrease of >4% in oxygen saturation. The apnea–hypopnea index (AHI) was defined as the average number of episodes of apnea and hypopnea per hour of sleep.[9,18,19] OSAS was defined as an AHI of >5 per hour during sleep in the presence of clinical symptoms suggesting OSAS. According to AHI, included patients were classified into 3 groups: mild group (group 1, AHI = 5–15, n = 24), moderate group (group 2, AHI = 16–30, n = 25), and severe group (group 3, AHI > 30, n = 20).
The exclusion criteria were as follows: patients with (1) congenital heart disease, (2) cardiac valve disease, (3) coronary artery diseases, (4) obstructive or restrictive lung disease demonstrated on pulmonary function test, (5) renal failure, (6) hepatic failure, (7) diabetes mellitus, (8) hypothyroidism and hyperthyroidism, (9) connective-tissue diseases, (10) pericardial disease, (11) atrial fibrillation, (12) permanent pacemaker, (13) smoking, (14) left ventricular (LV) ejection fraction < 50%, (15) hypertension (systolic blood pressure > 140 mm Hg and diastolic blood pressure > 90 mm Hg), and those (16) receiving medication.
2.2. Echocardiography
All patients underwent transthoracic echocardiography using a commercially available system (Acuson SC2000 Ultrasound system; Siemens Medical Solutions, CA) equipped with a transducer frequency of 3.5 MHz. Averages of 3 consecutive cycles were measured for all echocardiographic data. The acoustic acquisition mode was selected, and the patients were advised to hold breath to obtain high-quality images. The RV end-diastolic diameter (RVDD) was measured from the right ventricle-focused 4-chamber view obtained from the apical window at end-diastole. The RV anterior wall (RVAW) thickness was measured from the subcostal view using 2D imaging in diastole. The tricuspid annular plane systolic excursion (TAPSE) was measured by M-mod from the apical 4-chamber view. The percentage RV fraction of area change (FAC), defined as (end-diastolic area − end-systolic area)/end-diastolic area × 100%, was obtained by tracing the RV endocardium both in systole and diastole. The RV ejection fraction (RVEF) was measured by the biplane Simpson method.[13]
Standard grayscale 2D images were obtained at a frame rate of 70–90 frames/s from apical 4-chamber images. The angle between the Doppler beam and the wall was kept at <30°. Three cardiac cycles were stored for offline analysis using VVI. The RV endocardial border was traced manually from the annulus along the free wall to the apex, and then back to the annulus along the interventricular septum. The region of interest was adjusted to include the entire myocardial wall. The RV free wall (RVFW) was divided into 3 segments: basal, mid, and apical.[20] The epicardial border was automatically detected by the software. The peak systolic velocity, systolic ST, and STR of each segment were obtained.
To examine the intraobserver variability, the same observer examined the peak systolic velocity, ST, and STR in 10 random subjects 2 weeks after the first measurement. To examine the interobserver variability, a second independent blinded observer measured the same variables using the aforementioned described method.
2.3. Statistical analysis
All statistical data were analyzed using the SPSS version 18.0 software (SPSS Inc, IL) for Windows. The numeric variables were presented as mean ± standard deviation, and the categorical variables as percentages. The 1-way Kolmogorov–Smirnov test was conducted to test the normal distribution within groups. For ordinal parameters with a normal distribution, Student t test was used for comparison, whereas for parameters not distributed normally, nonparametric tests (such as Kruskal–Wallis for 3 or more groups and Mann–Whitney U test for 2 groups) were used to compare groups. A P value < 0.05 was considered significant.
3. Results
3.1. General information
The detailed clinical and demographic variables and AHI levels of the 69 patients are shown in Table 1. The variables age, sex, systolic blood pressure, and diastolic blood pressure showed no difference in the groups. The body mass index (BMI) was found to be higher in the OSAS groups than in the healthy group (t = 8.19, P = 0.04) (Figure 1); however, no differences were observed within the OSAS groups. The systolic pulmonary arterial pressure (SPAP) showed no differences between the control and mild groups, but it was higher in the moderate and severe groups compared with the control group (t = −4.187, P = 0.002; t = −5.162, P < 0.001, respectively).
Table 1.
Clinical and demographic characteristics and AHI levels of the groups.
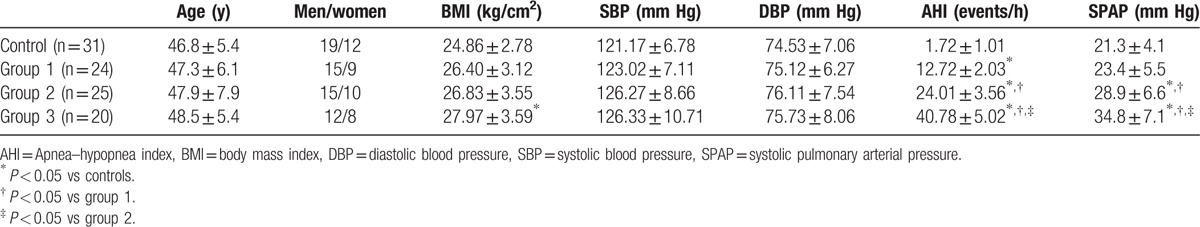
Figure 1.
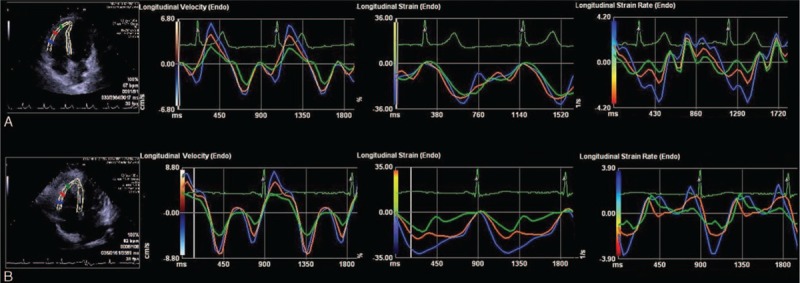
V, ST, and STR graphic result of VVI analysis of RV free wall in a healthy individual (A) and in a mild OSAS group patient (B). Obstructive sleep apnea patient (B) has lower ST, STR in apical segment, and higher V in basal segment compared to healthy individual (A). OSAS = obstructive sleep apnea syndrome, RV = right ventricular, ST = strain, STR = strain rate, V = velocity, VVI = velocity vector imaging.
3.2. Echocardiographic parameters
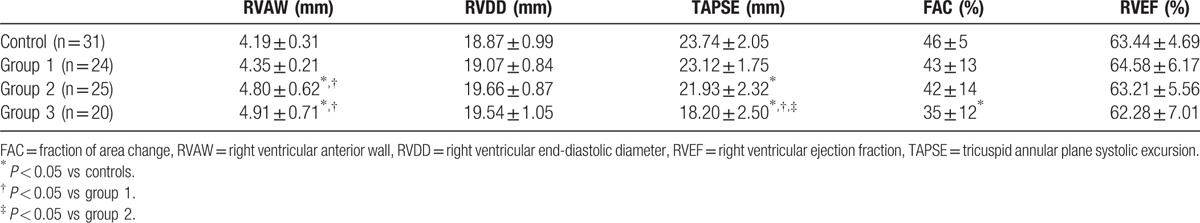
The conventional echocardiographic parameters of the patients in different groups are presented in Table 2. The RVAW values increased gradually with the severity of OSAS and were found to be higher in the severe groups than in the control and mild groups (t = 2.74, P = 0.022; t = 2.42, P = 0.041, respectively). The TAPSE values in the severe and moderate groups were lower than that in the control group (t = 2.86, P = 0.016; t = 12.46, P = 0.000, respectively) and had significant differences within the OSAS groups (severe group vs mild: t = 10.40, P = 0.000; severe group vs moderate: t = 7.27, P = 0.001, respectively). The FAC values were lower in the severe group than in the control group (t = 2.56, P = 0.043). The RVDD and RVEF values showed no differences among the groups.
Table 2.
Conventional echocardiographic parameters of the patients in different groups.
3.3. RV function using VVI
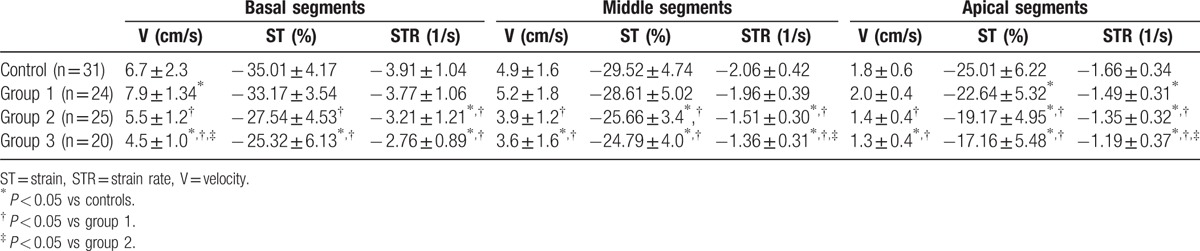
The VVI data of RVFW in different groups are presented in Table 3. In the mild OSAS group, the peak systolic velocity parameters in the RVFW basal segments increased (t = 2.216, P = 0.049), but no difference was found in the RVFW middle and apical segments compared with that in the control group. However, the values of peak systolic velocity reduced in the moderate group (t = 2.272, P = 0.044) and reduced more obviously in the severe group (t = 4.951, P = 0.001) compared with the control group. The values of systolic ST and STR of RVFW decreased in the apical segment of the mild OSAS group (t = 3.30, P = 0.02; t = 3.75, P = 0.01, respectively) and decreased obviously in all segments of the moderate and severe OSAS groups (moderate group vs control: ST: t = 4.77, P = 0.000, STR: t = 5.561, P = 0.000; severe group vs control: ST: t = 12.572, P = 0.000, STR: t = 6.491, P = 0.000, respectively).
Table 3.
VVI data of RVFW in different groups.
3.4. Interobserver and intraobserver variability
The interobserver variability was better for the peak systolic velocity [interclass correlation coefficient, 0.94; 95% confidence interval (CI), 0.87–0.97], ST (interclass correlation coefficient, 0.97; 95% CI, 0.94–0.99), and STR (interclass correlation coefficient, 0.93; 95% CI, 0.86–0.99) compared with that for TAPSE (interclass correlation coefficient, 0.86; 95% CI, 0.73–0.97). The intraobserver variability was better for the peak systolic velocity (interclass correlation coefficient, 0.93; 95% CI, 0.80–0.98), ST (interclass correlation coefficient, 0.95; 95% CI, 0.79–0.99), and STR (interclass correlation coefficient, 0.98; 95% CI, 0.88–0.99) compared with that for TAPSE (interclass correlation coefficient, 0.88; 95% CI, 0.62–0.94).
4. Discussion
OSAS is the most common form of sleep-disordered breathing characterized by repeated episodes of upper respiratory tract obstruction during sleep. It is usually associated with cardiovascular risk factors such as hypertension, coronary artery disease, and obesity. To determine the independent effects of OSAS-related hypoxemia and hemodynamic alterations on myocardial function, the aforementioned risk factors were excluded. Thus, no significant differences were found in age, sex, BMI, and blood pressure among the groups.
The structural organization of RV myocardial fibers is a characteristic arrangement, with most of the myocardial fibers oriented longitudinally and the circumferential layer poorly developed. For this reason, its contraction is mainly determined by longitudinal shortening.[21,22] Otherwise, the interventricular septum is shared by 2 ventricles, and mostly functions as part of the left ventricle. Therefore, this study analyzed the longitudinal systolic deformation and motion of the RVFW to represent the RV systolic function.
In this study, the changes in the VVI-derived parameters for RV systolic dysfunctions started from the mild OSAS group, whereas the changes in the conventional echocardiographic parameters such as RVAW, RVDD, TAPSE, and FAC began from the moderate or severe OSAS group. These results were similar to previous studies. Altekin et al[23] determined RV dysfunction in patients with OSAS using the STE method. They found that RV longitudinal systolic ST and STR decreased from the moderate OSAS group, indicating that the changes in the deformation parameters started before those in the conventional echocardiographic parameters. However, the regional myocardial dysfunction has not been fully elucidated. The present study found that the changes in the myocardial systolic function started in the mild OSAS group; moreover, the changes in the segments were different. The peak systolic velocities increased only in the RVFW basal segments, and the systolic ST and STR decreased in apical segments. This difference might be due to the different anatomical structures of the segments. Anatomically, the RVFW basal segment is characterized by a smoother myocardial wall and has a greater fiber shortening, which acts as a major contributor to the overall RV systolic performance.[24] In patients with OSAS, respiration efforts against the increased upper airway resistance increase the intrathoracic negative pressure and venous return, leading to increased RV preload. In the early stage of OSAS, the myocardium is compensated by increased cardiac contractility according to the Frank–Starling mechanism. At this time, the contractility of the basal segment, the major systolic contributor, had increased susceptibility. As a result, the systolic velocity increased.[24]
However, the apical segment was thinner than the basal segment and had more trabecular muscles. Therefore, it was susceptible to impairment when the volume or pressure was overloaded. Previous studies showed similar results. Dambrauskaite et al[25] evaluated regional RV dysfunction in patients with chronic pulmonary hypertension using 2D-STI. They observed that the deformation of the apical segment of RVFW was affected more than the basal smooth segment of the right ventricle. In addition, different alterations between segments might be associated with different wall stresses. Kepez et al[26] found that the effect of stress on the RVFW was different: a thinner apical segment was exposed to a greater wall stress in the pressure-overloaded right ventricle.
In the moderate group, the systolic velocity and TAPSE decreased and RVAW increased. The values of ST and STR decreased with the severity of the disease. These results indicated the deterioration of RV dysfunction. In OSAS, the large intrathoracic negative pressure swings against an occluded airway cause not only increased venous return and volume overload but also expanding and remodeling of the right ventricle.[23] In addition, repetitive nocturnal arterial oxygen desaturation and hypercapnia increase in pulmonary artery pressure (PAP)[27] and also lead to RV dysfunction. The degree of PAP increased in OSAS in previous studies. Bayram et al[15] found a moderate PAP increase in 20% to 40% of OSAS cases and a correlation existed between PAP levels and RV functions. Similar findings were observed in the present study. The SPAP degree also increased from the moderate group in 19 patients with OSAS (27.5%). The pulmonary vascular endothelium remodeling and vasoconstriction that arise in the hypoxic and hypercapnic stages may lead to a gradual and continuous development of PAP. Forced right ventricle contraction was caused by the volume and pressure overload, and also the structural remodeling increased myocardial oxygen demands. However, the blood inflow of the coronary artery reduced due to the vascular endothelium remodeling and vasoconstriction. Insufficient supply and increased demand may cause RV ischemia and further deteriorate the RV function.[28] In addition, LV dysfunction induced by OSAS may lead to RV dysfunction, mainly because of the close anatomical association between the 2 ventricles.[29]
4.1. Limitations
A technical limitation in the present study was that the VVI method was depended on the B-mode image quality, which was affected by the presence of obesity or pulmonary conditions in patients with OSAS. The image quality could be improved by breath holding and selecting acoustic mood to acquire images. Second, the function of interventricular septum was not evaluated, as it was considered a part of the LV function. The RV systolic function was a result of synergistic action of the RVFW and septum, although the septum only plays a small role in RV contraction. Third, the principals of the present study were relatively small. A larger longitudinal study would be necessary to confirm the results obtained in this study. Moreover, patients with good acoustic windows were chosen, and patients with OSAS with significant comorbidities were excluded. As a result, the conclusion may not be generalized to all patients, especially those with overt comorbidities that could influence the measurements.
5. Conclusion
OSAS itself may induce RV dysfunction, even in the absence of comorbidities that could act as confounding factors on RV functions. In the early stage of OSAS, changes in myocardial motion and deformation start before the development of RV failure and pulmonary hypertension. The alterations of regional RVFW systolic function include an increase in systolic velocities in the basal segment and a decrease in deformation in the apical segment. The VVI method is a unique technology demonstrating not only myocardial velocity but also deformation, which presents a new approach to detect myocardial dysfunction at the early phase of OSAS before alterations in other parameters. Therefore, the VVI method is expected to be a worthy technique for early clinical therapy to prevent the progression of heart failure.
Footnotes
Abbreviations: AHI = apnea–hypopnea index, BMI = body mass index, FAC = fraction of area change, OSAS = obstructive sleep apnea syndrome, RV = right ventricular, RVAW = RV anterior wall, RVDD = RV end-diastolic diameter, RVEF = RV ejection fraction, RVFW = right ventricular free wall, SPAP = systolic pulmonary arterial pressure, ST = strain, STR = strain rate, TAPSE = tricuspid annular plane systolic excursion, V = velocity, VVI = velocity vector imaging.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 2014; 383:736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vitarelli A, Terzano C, Saponara M, et al. Assessment of right ventricular function in obstructive sleep apnea syndrome and effects of continuous positive airway pressure therapy: a pilot study. Can J Cardiol 2015; 31:823–831. [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parati G, Lombardi C, Hedner J, et al. Position paper on the management of patients with obstructive sleep apnea and hypertension: joint recommendations by the European Society of Hypertension, by the European Respiratory Society and by the members of European COST (Cooperation in Scientific and Technological research) ACTION B26 on obstructive sleep apnea. J Hypertens 2012; 30:633–646. [DOI] [PubMed] [Google Scholar]
- 5.Gaisl T, Bratton DJ, Kohler M. The impact of obstructive sleep apnoea on the aorta. Eur Respir J 2015; 46:532–544. [DOI] [PubMed] [Google Scholar]
- 6.Rossi VA, Stradling JR, Kohler M. Effects of obstructive sleep apnoea on heart rhythm. Eur Respir J 2013; 41:1439–1451. [DOI] [PubMed] [Google Scholar]
- 7.Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol 2010; 7:677–685. [DOI] [PubMed] [Google Scholar]
- 8.D’Andrea A, Martone F, Liccardo B, et al. Acute and chronic effects of noninvasive ventilation on left and right myocardial function in patients with obstructive sleep apnea syndrome: a speckle tracking echocardiographic study. Echocardiography 2016; 33:1144–1155. [DOI] [PubMed] [Google Scholar]
- 9.Barbe F, Duran-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med 2010; 181:718–726. [DOI] [PubMed] [Google Scholar]
- 10.Raisinghani A, Jen R, Wilson J, et al. Obstructive sleep apnea effects on the right ventricle and beyond. Can J Cardiol 2015; 31:821–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haji SA, Movahed A. Right ventricular infarction–diagnosis and treatment. Clin Cardiol 2000; 23:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naderi N, Ojaghi Haghighi Z, Amin A, et al. Utility of right ventricular strain imaging in predicting pulmonary vascular resistance in patients with pulmonary hypertension. Congest Heart Fail 2013; 19:116–122. [DOI] [PubMed] [Google Scholar]
- 13.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23:685–713.quiz 786-688. [DOI] [PubMed] [Google Scholar]
- 14.Lindqvist P, Calcutteea A, Henein M. Echocardiography in the assessment of right heart function. Eur J Echocardiogr 2008; 9:225–234. [DOI] [PubMed] [Google Scholar]
- 15.Bayram NA, Ciftci B, Bayram H, et al. Effects of continuous positive airway pressure therapy on right ventricular function assessment by tissue Doppler imaging in patients with obstructive sleep apnea syndrome. Echocardiography 2008; 25:1071–1078. [DOI] [PubMed] [Google Scholar]
- 16.Jurcut R, Giusca S, La Gerche A, et al. The echocardiographic assessment of the right ventricle: what to do in 2010? Eur J Echocardiogr 2010; 11:81–96. [DOI] [PubMed] [Google Scholar]
- 17.Park JH, Kusunose K, Motoki H, et al. Assessment of right ventricular longitudinal strain in patients with ischemic cardiomyopathy: head-to-head comparison between two-dimensional speckle-based strain and velocity vector imaging using volumetric assessment by cardiac magnetic resonance as a “gold standard”. Echocardiography 2015; 32:956–965. [DOI] [PubMed] [Google Scholar]
- 18.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009; 5:263–276. [PMC free article] [PubMed] [Google Scholar]
- 19.Punjabi NM, Newman AB, Young TB, et al. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med 2008; 177:1150–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motoji Y, Tanaka H, Fukuda Y, et al. Efficacy of right ventricular free-wall longitudinal speckle-tracking strain for predicting long-term outcome in patients with pulmonary hypertension. Circ J 2013; 77:756–763. [DOI] [PubMed] [Google Scholar]
- 21.Haddad F, Hunt SA, Rosenthal DN, et al. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008; 117:1436–1448. [DOI] [PubMed] [Google Scholar]
- 22.Vitarelli A, Terzano C. Do we have two hearts? New insights in right ventricular function supported by myocardial imaging echocardiography. Heart Fail Rev 2010; 15:39–61. [DOI] [PubMed] [Google Scholar]
- 23.Altekin RE, Karakas MS, Yanikoglu A, et al. Determination of right ventricular dysfunction using the speckle tracking echocardiography method in patients with obstructive sleep apnea. Cardiol J 2012; 19:130–139. [DOI] [PubMed] [Google Scholar]
- 24.Geva T, Powell AJ, Crawford EC, et al. Evaluation of regional differences in right ventricular systolic function by acoustic quantification echocardiography and cine magnetic resonance imaging. Circulation 1998; 98:339–345. [DOI] [PubMed] [Google Scholar]
- 25.Dambrauskaite V, Delcroix M, Claus P, et al. Regional right ventricular dysfunction in chronic pulmonary hypertension. J Am Soc Echocardiogr 2007; 20:1172–1180. [DOI] [PubMed] [Google Scholar]
- 26.Kepez A, Niksarlioglu EY, Hazirolan T, et al. Early myocardial functional alterations in patients with obstructive sleep apnea syndrome. Echocardiography 2009; 26:388–396. [DOI] [PubMed] [Google Scholar]
- 27.Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis 2009; 51:363–370. [DOI] [PubMed] [Google Scholar]
- 28.Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57:119–127. [DOI] [PubMed] [Google Scholar]
- 29.Mittal SR, Barar RV, Arora H. Echocardiographic evaluation of left and right ventricular function in mild hypertension. Int J Cardiovasc Imaging 2001; 17:263–270. [DOI] [PubMed] [Google Scholar]


