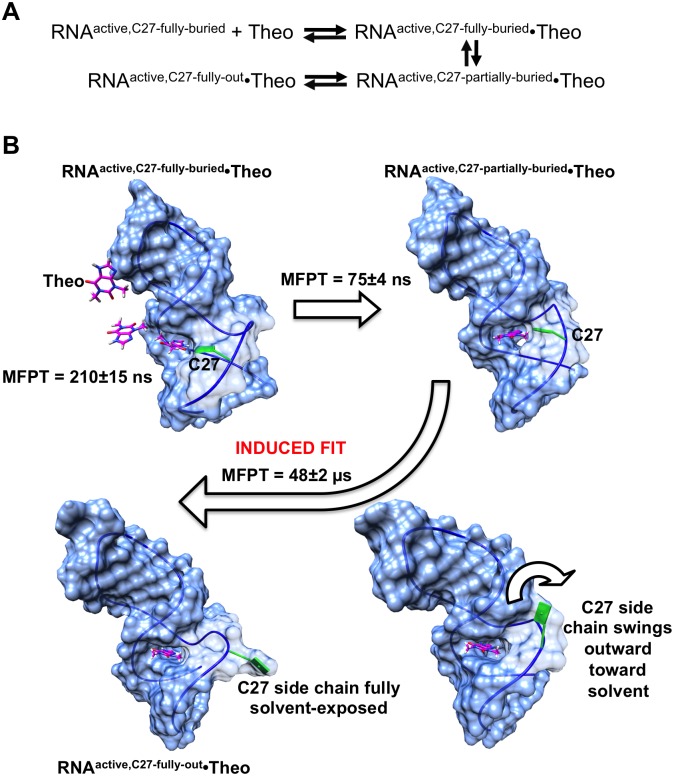
Fig 11. Complete modeled theophylline binding pathway.
(A) Schematic showing the main intermediate states between initial diffusion of theophylline into the RNA binding site (RNAactive,C27-fully-buried + Theo) and the final, fully associated RNA–theophylline complex observed in the NMR structure (RNAactive,C27-fully-out•Theo). (B) Molecular view of the binding pathway. Mean first-passage times (MFPT) required to transition between consecutive intermediate states are shown. The conformational transition from the RNAactive,C27-partially-buried•Theo state, in which theophylline is bound within a non-optimal binding pocket, to the state RNAactive,C27-fully-out•Theo, in which the binding pocket has reached its final, optimal conformation, is characteristic of an induced fit process. This induced fit follows the previously known conformational selection binding mechanism. Theophylline and the C27 base are colored magenta and green, respectively.