Abstract
Background:
Prostaglandin E1 (PGE1) is widely used as a pretreatment for myocardial reperfusion injury in animal experiments. However, the cardioprotective effects of PGE1 in patients have not been established. We performed a meta-analysis to investigate whether PGE1 is cardioprotective, based on the reduction of correlative reperfusion injury events (CRIE), major adverse cardiac events (MACE), and biomarker release in patients with ischemia reperfusion injury.
Methods:
The Medline, EMBASE, and Cochrane databases were searched for randomized clinical trials confirming the effects of PGE1. Two investigators independently selected suitable trials, assessed trial quality, and extracted data.
Results:
Six studies in patients undergoing percutaneous coronary intervention (4 studies) and cardiac surgery (2 studies), comprising a total of 445 patients, were included in this review. The results showed that PGE1 reduced the incidence of CRIE (relative ratio 0.4 [95% confidence interval 0.43, 0.95]), the incidence of MACE (0.35 [0.17, 0.70]), and the level of troponin T (standardized mean difference 20.28 [20.47, 20.09]), creatine kinase-MB (−1.74 [−3.21, − 0.27]), interleukin-6 (−1.37 [−2.69, − 0.04]), and interleukin-8 (−2.05 [−2.75, − 1.34]).
Conclusion:
PGE1 may have beneficial effects on myocardial reperfusion injury in the clinic.
Keywords: correlative reperfusion injury events, major adverse cardiac events, Prostaglandin E1, reperfusion injury
1. Introduction
Ischemic heart disease (IHD) is a common cardiovascular disease, both in developed and developing countries. In 2011, a total of 7 million people died of IHD globally and it has become the leading cause of mortality according to a report by the World Health Organization.[1] The primary objective in the treatment of IHD is to restore blood perfusion to ensure adequate supply of tissue oxygen and nutrients and then to prevent ischemic injury. However, several clinical and animal studies have found that, when low tissue perfusion after ischemia is addressed, not only does ischemic tissue damage fail to resolve but also the injury is aggravated.[2–8] This phenomenon, first described by Jennings et al[9] in 1960, is defined as ischemic reperfusion injury (IRI). Myocardial IRI is often found in patients undergoing procedures involving ischemia reperfusion such as percutaneous coronary intervention (PCI), thrombolysis, coronary artery bypass grafting (CABG), and valve replacement, and its primary clinical manifestations are ventricular arrhythmia, lack of reflow phenomenon, and distal embolization.[10–12] During reperfusion, inflammatory, and oxidative stress injury causes white blood cells to release inflammatory mediators such as interleukins and to activate complement, leading to myocardial injury, and endothelial injury activates platelet resulting in microvasculature blockage.[13,14] Moreover, ventricular fibrillation or heart failure (HF) also arises from inner cell membrane instability.[15]
Prostaglandin E1(PGE1), also known as alprostadil, has many physiological and pharmacological properties which may contribute to IRI. In recent decades, some animal and clinical studies have shown that PGE1 can improve reperfusion injury (RI) in several biological systems.[16–19] However, it is difficult to conclude that PGE1 has a beneficial effect in myocardial RI because of the limited numbers of patients included in these studies. Therefore, the purpose of the present study was to identify and combine all relevant published clinical randomized controlled trials (RCTs) to investigate the effects of PGE1 on myocardial RI.
2. Methods
2.1. Data sources
The Medline, EMBASE, Cochrane, and databases and the lists of references found in original and review articles were searched independently by 2 reviewers (Zhu, Xu) using medical subject heading terms, key words, titles, and abstracts. The search keywords were “myocardial reperfusion Injury,” “myocardial injury,” “reperfusion injury,” and “myocardial reperfusion” paired with “alprostadil,” “prostaglandin E1,” and “PGE1.” All historical literature was searched up until December 2016, and the search was not limited to the English language.
2.2. Study selection
An initial eligibility screen of all retrieved titles and abstracts was conducted, and original studies were included in our meta-analysis if they met the following criteria: (1) included human subjects; (2) included patients with myocardial ischemia who were randomly assigned to receive either PGE1 or placebo; (3) PGE1 was administrated after ischemia and before reperfusion; (4) included sufficient data on correlative reperfusion injury events (CRIE), major adverse cardiac events (MACE) or relevant biomarkers (data at baseline and at the end of the study and/or data on change in standardized mean difference (SMD) from baseline or appropriate data estimation). Full manuscripts were obtained for all selected articles based on the assessment of abstracts. Only fully published trials were included (abstracts and congress presentations were not included). Primary outcome was the combined endpoint, divided into 2 subgroups: (1) CRIE, including no-reflow phenomenon, acute thrombus formation, and thrombolysis in myocardial infarction (TIMI) flow< 3; and (2) MACE, including recurrent angina, myocardial infarction, HF, and target vessel revascularization (TVR). Troponin T (TNT), creatine kinase-MB (CK-MB), interleukin-6 (IL-6), and interleukin-8 (IL-8) were defined as secondary outcome parameters. Trials not reporting any of these parameters were excluded from the review. Two investigators independently reviewed all full-text articles that could possibly meet the inclusion criteria according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement[20] and the Cochrane Handbook guidelines.[21] In the case of disagreement, consensus was obtained by discussion with a third author (Huang).
2.3. Data extraction
All selected papers were reviewed by 2 reviewers (Zhu, Xu), who independently extracted data to a data sheet. Data extraction included year of publication, study design, sample size, patient characteristics, inclusion and exclusion criteria, control and intervention protocol, randomization, blinding, and follow-up, as well as the outcome parameters described previously. Where the timing of when events took place differed, the primary outcome was divided into 2 subgroups: (1) CRIE during surgery and (2) MACE during hospitalization and after discharge. With respect to biomarker data, we used the peak values as reported in the paper. The SMD was used for analysis as detection times and unit differed. Where data were presented in a graph but not in the text, we request the data from the corresponding author of the paper. If the data were not provided, we extrapolated them from the graph using a charting digital tool (GetData Graph Digitizer, http://getdata-graph-digitizer.com). Following the extraction of relevant data by the 2 authors, data were examined for possible inconsistencies which were then resolved by discussion, and if consensus could not be reached, a third author was consulted (Huang). Studies were not conducted directly on humans and ethical approval was therefore not necessary.
2.4. Quality assessment
Two authors used the 5 domains of the Cochrane risk of bias tool to evaluate the quality of the included studies, using the following criteria: randomization sequence generation, concealment of randomization sequence, blinding of intervention, blinding of outcome assessment, and incomplete outcome reporting, and studies were classified as having low risk, high risk, or unclear risk of bias for each item, as suggested in the Cochrane Handbook.[21]
2.5. Statistical analysis
The verified data were analyzed using Stata software (version 13.0; Stata Corporation, College Station, TX) and REVMAN software (version 5.2; Cochrane Collaboration, Oxford, UK). Zhu entered the data and Xu verified data entry. The secondary outcome of this meta-analysis was the percentage change in biomarkers between the baseline and the final level in response to PGE1 administration. The relative ratio (RR) and SMD, and their corresponding 95% confidence intervals (CI), were calculated for dichotomous or continuous outcome data, respectively. A fixed effects model was used to analyze data with values higher than 0.10 by heterogeneity testing (χ2-based Q-test), whereas a random effects model was used when values were less than 0.10. The magnitude of heterogeneity was assessed by I2 test (I2 = 0–25%, no heterogeneity; I2 = 25–50%, moderate heterogeneity; I2 = 50–75%, large heterogeneity; I2 = 75–100%, extreme heterogeneity). An intervention was assumed to have had a significant if the 95% CI did not include the value 1 for RR or 0 for SMD. In the analysis for small-study effects, publication bias was assessed using funnel plot techniques and Harbord's test.[22]
3. Results
3.1. Literature search
The literature search identified 109 records of clinical trials in Medline, 212 records in EMBASE, and 22 records in the Cochrane databases (Fig. 1), as well as 9 additional abstracts identified from the reference lists of relevant papers. After checking for duplicates, 81 unique references remained and 73 of them were excluded for reasons such as irrelevance to myocardial reperfusion injury or describing a nonclinical trial; the remaining 8 full texts underwent further evaluation. Among these, 2 articles[23,24] were excluded as they did not provide the necessary data for our meta-analysis.
Figure 1.
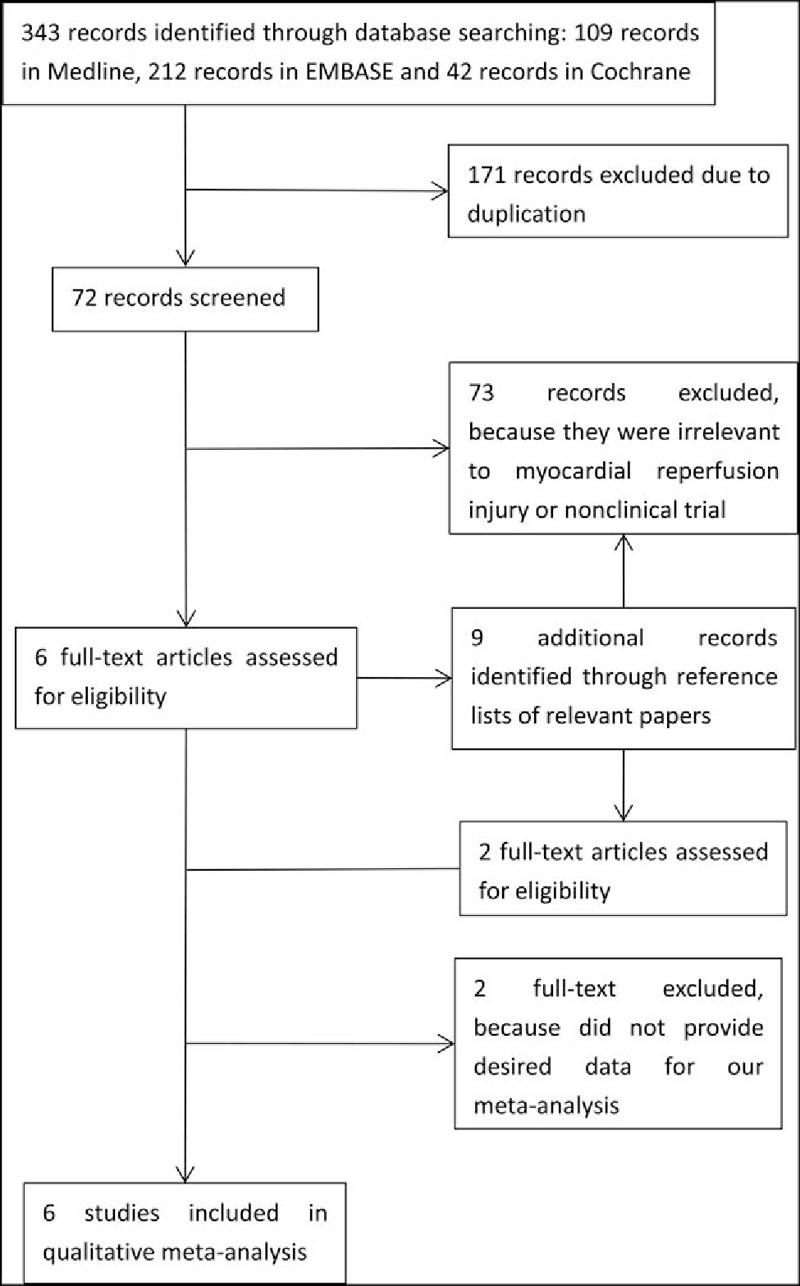
Flow diagram of study search and selection.
3.2. Surgical procedures in selected studies
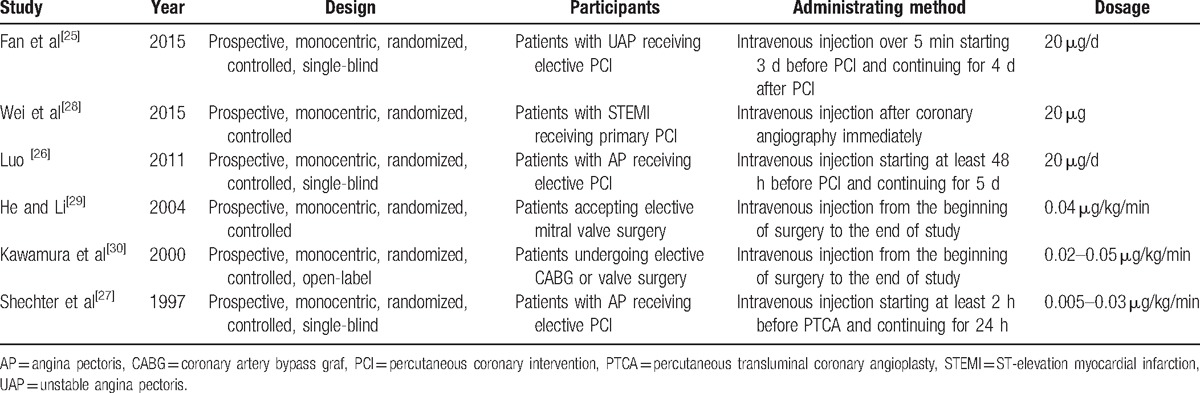
Of the 6 included studies, 3 involved patients receiving elective PCI,[25–27] 1 involved primary PCI,[28] 1 involved elective mitral valve surgery,[29] and 1 involved elective CABG or valve surgery[30] (Table 1).
Table 1.
Study design characteristics.
3.3. PGE1 protocols
PGE1 was administrated by intravenous injection in all studies (Table 1). In 2 studies,[29,30] patients were administered PGE1 at a dose of 0.02 to 0.05 μg/kg/min from the beginning of surgery to the end of the study. In 2 other studies,[25,26] patients were administered 20 μg/day PGE1 starting at least 48 hours before PCI and continuing for 5 days or starting 3 days before PCI and continuing for 4 days after PCI, respectively. Moreover, one[28] study described the administration of 20 μg PGE1 immediately after coronary angiography and another[27] administered 0.005 to 0.03 μg/kg/min PGE1, starting at least 2 hours before PCI and continuing for 24 hours.
3.4. Patient characteristics
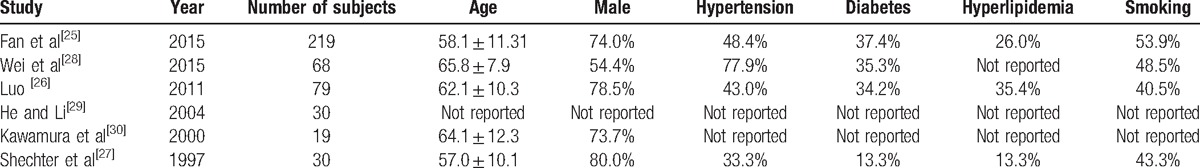
The mean ages of included patients ranged from 58.1 to 65.8 years (Table 2), the percentages of males varied from 54.4% to 80.0%, and the percentages of smokers ranged from 40.5% to 53.9%. Moreover, the percentages varied from 33.3% to 77.9%, 13.3% to 37.4%, and 13.3% to 35.4% for patients with hypertension, diabetes, and hyperlipidemia, respectively.
Table 2.
Patient characteristics.
3.5. Primary outcome
The primary outcome included 2 subgroups: CRIE and MACE. The PGE1 group had a reduced incidence of CRIE (RR 0.40 [95%CI 0.43, 0.95]) (Fig. 2) compared with control, as reported in 3 studies,[24,25,27] and there was no evidence for statistical heterogeneity (χ2 = 2.91, I2 = 0%, and Pheterogeneity = .878). MACE was reported in 3 studies[24,26,27] and the results suggested that PGE1 reduced the incidence of MACE compared with placebo (0.35 [0.17, 0.70]), with no statistical heterogeneity (χ2 = 1.24, I2 = 0%, and Pheterogeneity = .978). Overall effects also showed that PGE1 was associated with lower risk of the primary outcome (0.53 [0.36,0.79]), and no statistical heterogeneity was found (χ2 = 5.53, I2 = 0%, and Pheterogeneity = .954).
Figure 2.
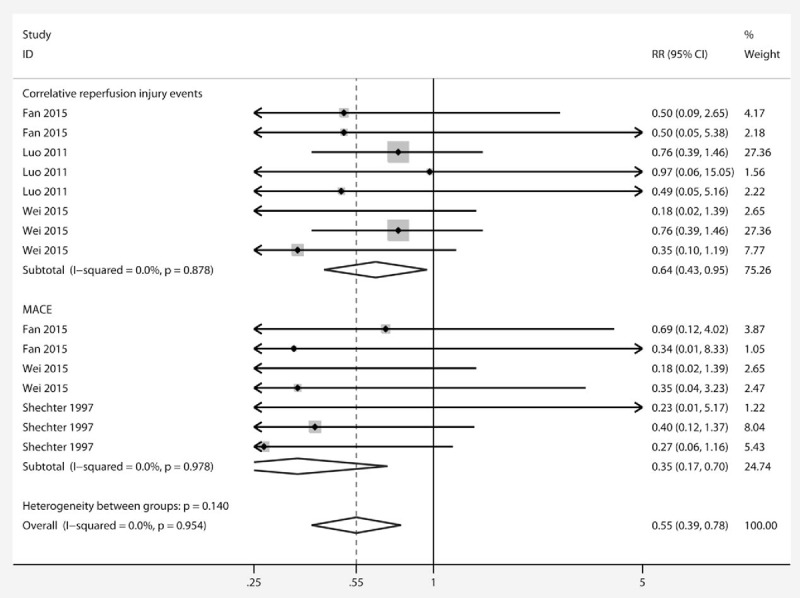
Forest plot showing the effects of PGE1 on incidence of correlative reperfusion injury events and MACE. A significant effect of PGE1 was assumed if the 95% CI did not include the value 1 for RR. CI = confidence interval, MACE = major adverse cardiac events, PGE1 = prostaglandin E1, RR = risk ratio.
3.6. Secondary outcome
TNT was the most widely reported biomarker of myocardial injury and was significantly reduced by PGE1 treatment (SMD −1.74 [95%CI −3.21, −0.27]) (Fig. 3) compared with control, although significant statistical heterogeneity was observed (χ2 = 16.27, I2 = 89%, and Pheterogeneity = 0). The levels of CK-MB in 3 studies also showed significant differences between the groups (−1.64 [−3.00, −0.28]), and statistical heterogeneity was observed (χ2 = 14.42, I2 = 86%, and Pheterogeneity = 0).
Figure 3.
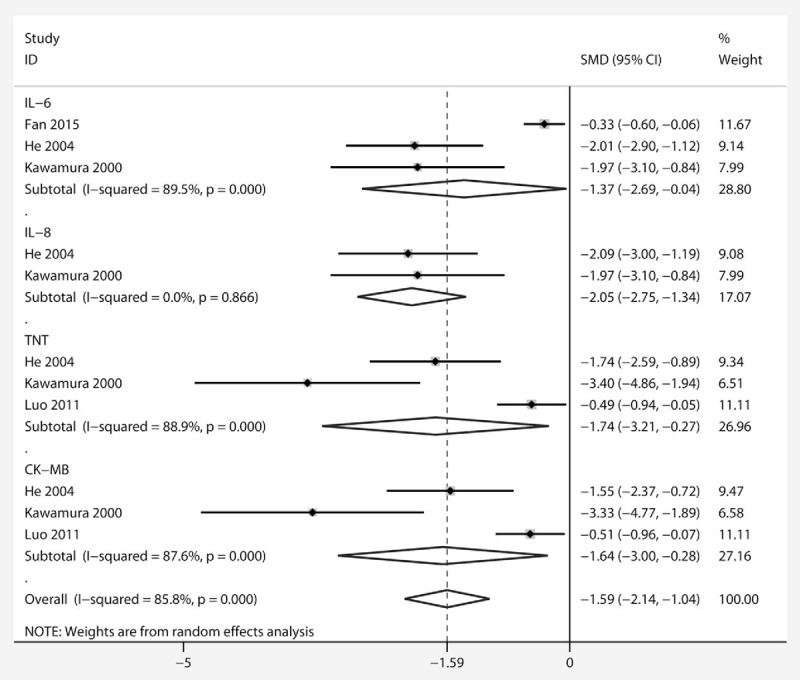
Forest plot showing the effect of PGE1 on changes in TNT, CK-MB, IL-6, and IL-8 levels. A significant effect of PGE1 was assumed if the 95% CI did not include the value 0 for SMD. CK-MB = creatine kinase-MB, IL-6 = interleukin-6, IL-8 = interleukin-8, PGE1 = prostaglandin E1, SMD = standardized mean difference, TNT = troponin T.
Regarding the expression of inflammatory response markers, IL-6 was reported in 3 studies and IL-8 in 2, and both markers were reduced with PGE1 treatment (−1.37 [−2.69, −0.04]; −2.05 [−2.75, −1.34], respectively). Overall effects also showed that PGE1 lowered the secondary outcome parameters compared with control (−1.59 [−2.14, −1.04]), but an obvious heterogeneity was observed (χ2 = 63.98, I2 = 85.8%, and Pheterogeneity = 0).
3.7. Quality of studies
In 4 studies, the process of sequence generation was correctly performed[25,26,28,29] (Fig. 4), but in the other studies, the methods were not described in detail. In 2 studies concealment was achieved using a random number table,[28,29] which was defined as high risk of bias. One study used an open trial design, 3 adopted single blinding, and the other 2 studies did not describe the blinding method; none of the studies contained sufficient information to judge the risk of performance and detection bias. One study exhibited withdrawal bias,[25] but missing data did not affect the analysis so this study was defined as low risk.
Figure 4.
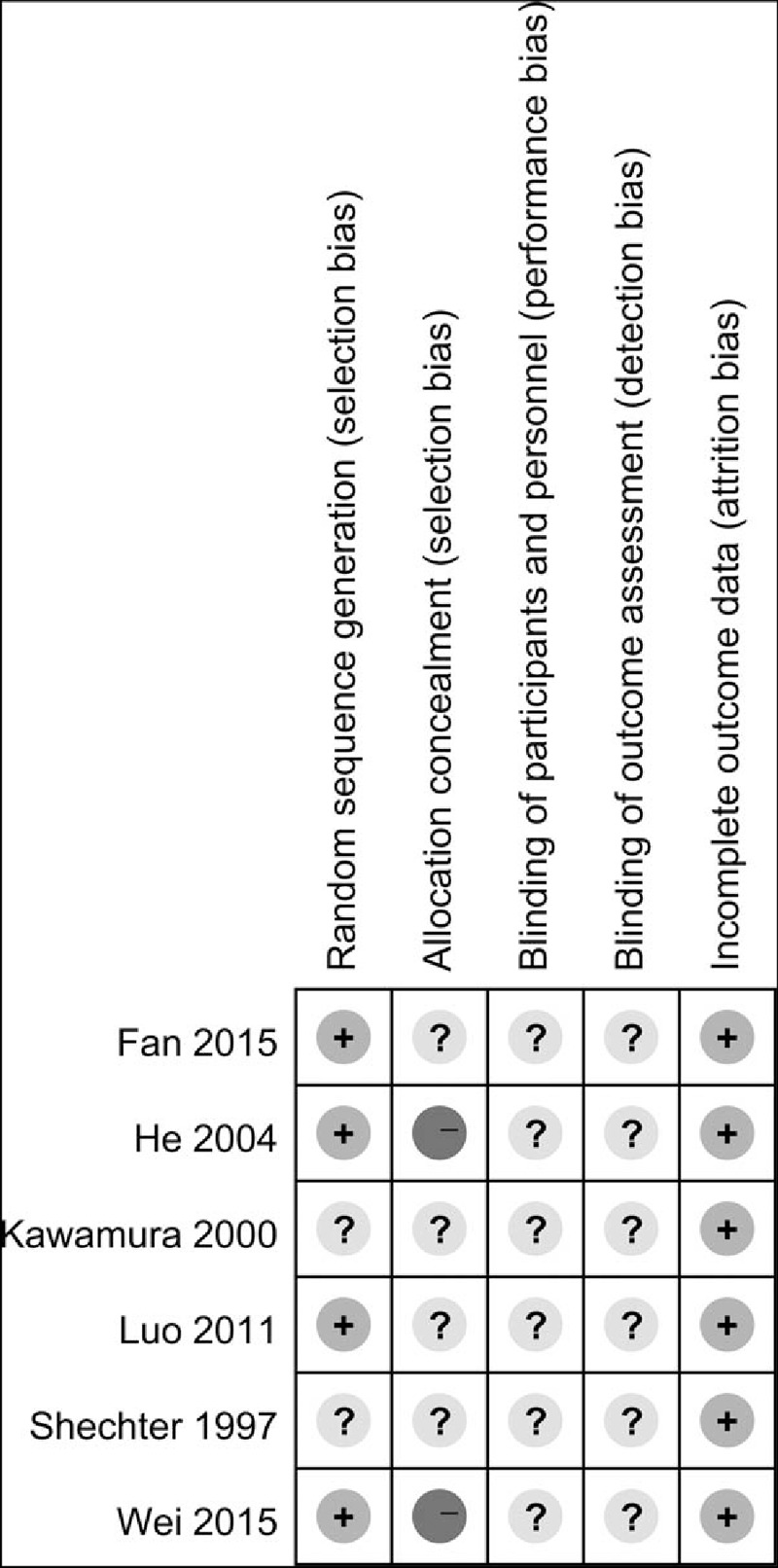
Risk of bias summary. + = low risk, − = high risk, ? = unclear risk.
3.8. Publication bias
Funnel plots of the study were visually symmetric and a statistical analysis of funnel plots also suggested that no publication bias was present (Harbord's test, P = .34) (Fig. 5).
Figure 5.
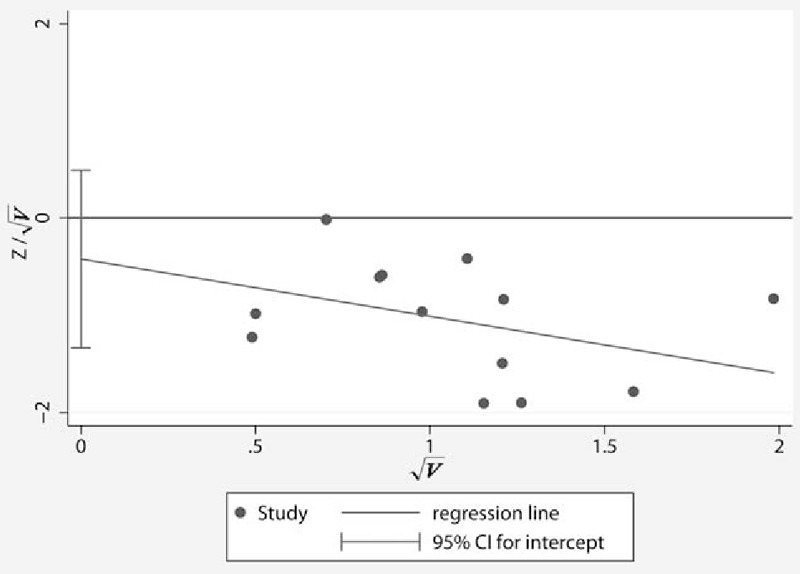
Harbord's funnel plot to evaluate publication bias in the effects of PGE1 on incidence of CRIE and MACE. CRIE = correlative reperfusion injury events, MACE = major adverse cardiac events, PGE1 = prostaglandin E1.
4. Discussion
We conducted a review of the protective effects of PGE1 on myocardial RI in the clinical setting using data from 6 RCTs comprising a total of 445 patients. To our knowledge, this meta-analysis is the first review of the effects of PGE1 on RI, although relatively few studies were included in our review. The objective of our study was to confirm whether PGE1 could protect the heart from myocardial RI by investigating the incidence of CRIE and MACE in the identified studies.
The main finding of this meta-analysis was that PGE1 treatment significantly reduced the incidence of CRIE and MACE. Moreover, the secondary outcome parameters TNT, CK-MB, IL-6, and IL-8 were also reduced by PGE1, although there were some limitations to these conclusions.
Four studies focused on the effect of PGE1 on biomarker and inflammatory factor levels, so short or long-term clinical outcomes were not the primary outcome. Some studies did not report on the occurrence of adverse events, such as death. Therefore, some low probability adverse events were zero in both the PGE1 and placebo groups, which required us to exclude these events when calculating the RR. It is therefore inevitable that we may have over-estimated the possible effect because of the exclusion of patients with no events. Although our analysis suggested that PGE1 reduced the primary outcome, we used the definition applied by the investigators of the respective studies when examining the endpoint events. This means that different events could have been classified as identical, and we may have erroneously pooled these events in the meta-analysis. In addition, the results of the meta-analysis are largely driven by the study of Fan et al,[25] which may have induced bias.
TNT and CK-MB are commonly used as biomarkers for myocardial injury, and IL-6 and IL-8 are also thought to be highly expressed in reperfusion injury.[31–34] In this analysis, we therefore used these biomarkers as secondary endpoints to determine the molecular biological effects of PGE1 in RI. We selected the peak values of these biomarkers in their respective studies, but this nonetheless led to inevitable variance as the reported time-points differed between studies, even in comparable settings. Statistical heterogeneity was therefore observed for all biomarkers except IL-8. Although we intended to perform subgroup analysis to explore heterogeneity, the number of included studies was too few. Additionally, we included studies with significant clinical heterogeneity. Studies differed in setting, patient population, and extent of ischemia the patients were at risk for. Patients undergoing elective or primary PCI, CABG, and valve surgery were all pooled for the meta-analysis on the chosen outcome parameters. Because of the existence of clinical and statistical heterogeneity, we used a random effects model for the secondary endpoint. Since random effects models typically provide a broader confidence interval than fixed effects models, they may therefore draw a more conservative conclusion.[35]
An important limitation of most of these studies was adequate blinding design, which is seemingly more difficult to achieve than in trials investigating a pharmacological agent. In the present meta-analysis, unfortunately, all included studies presented a risk of blinding bias.
Overall, our meta-analysis indicated that PGE1 treatment prior to myocardial reperfusion appeared to be superior to placebo in reducing CRIE, MACE, and the levels of related biomarkers. However, this conclusion is based on studies in a small group of patients, and this restriction in available data ensured that no further subgroup analysis could be performed in patients with PCI, valve surgery, or CABG. Moreover, inadequate blinding design may also have led to false positives in the data. Therefore, additional, large RCTs are required to firmly establish the role of PGE1 in regulating myocardial reperfusion injury.
Footnotes
Abbreviations: CABG = coronary artery bypass grafting, CK-MB = creatine kinase-MB, CRIE = correlative reperfusion injury events, HF = heart failure, IHD = ischemic heart disease, IL-6 = interleukin-6, IL-8 = interleukin-8, IRI = ischemic reperfusion injury, MACE = major adverse cardiac events, PCI = percutaneous coronary intervention, PGE1 = prostaglandin E1, RCT = randomized controlled trial, RI = reperfusion injury, RR = relative ratio, SMD = standardized mean difference, TIMI = thrombolysis in myocardial infarction, TNT = troponin T, TVR = target vessel revascularization.
HZ and XX contributed equally to this study.
Authorship: Designed the study: JH, LZ, and HZ. Performed the study: HZ and XX. Analyzed the data: HZ, XX, and JH. Wrote the paper: HZ, XX, and YD. Revised the article: LZ, YD, and JH.
Funding: This study was supported by Beijing Lisheng Cardiovascular Health Foundation of China (LSG1501132) and Zhejiang Bureau of Science and Technology(2017C37130).
The authors have no conflicts of interest to disclose.
References
- [1].Globocan 2013: World Health Organisation released 10 lethal factor in which Ischemic heart disease listed in the first. Available at: http://www.un.org/chinese/News/story.asp?NewsID=20179. Accessed December 7, 2016. [Google Scholar]
- [2].Kamat P, Vandenberghe S, Christen S, et al. Dexrazoxane shows no protective effect in the acute phase of reperfusion during myocardial infarction in pigs. PLoS One 2016;11:e168541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen WR, Chen YD, Tian F, et al. Effects of liraglutide on reperfusion injury in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Imaging 2016;9:pii: e005146. [DOI] [PubMed] [Google Scholar]
- [4].Hartman MH, Vreeswijk-Baudoin I, Groot HE, et al. Inhibition of interleukin-6 receptor in a murine model of myocardial ischemia-reperfusion. PLoS One 2016;11:e167195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen Y, Ba L, Huang W, et al. Role of carvacrol in cardioprotection against myocardial ischemia/reperfusion injury in rats through activation of MAPK/ERK and Akt/eNOS signaling pathways. Eur J Pharmacol 2016;796:90–100. [DOI] [PubMed] [Google Scholar]
- [6].Mudaliar H, Rayner B, Billah M, et al. Remote ischemic preconditioning attenuates EGR-1 expression following myocardial ischemia reperfusion injury through activation of the JAK-STAT pathway. Int J Cardiol 2016;228:729–41. [DOI] [PubMed] [Google Scholar]
- [7].Hamarneh A, Sivaraman V, Bulluck H, et al. The effect of remote ischemic conditioning and glyceryl trinitrate on perioperative myocardial injury in cardiac bypass surgery patients: rationale and design of the ERIC-GTN study. Clin Cardiol 2015;38:641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kimura K, Nakao K, Shibata Y, et al. Randomized controlled trial of TY-51924, a novel hydrophilic NHE inhibitor, in acute myocardial infarction. J Cardiol 2016;67:307–13. [DOI] [PubMed] [Google Scholar]
- [9].Jennings R, Sommers H, Smyth G. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol 1960;70:68–78. [PubMed] [Google Scholar]
- [10].Samantaray A, Chandra A, Panigrahi S. Amiodarone for the prevention of reperfusion ventricular fibrillation. J Cardiothorac Vasc Anesth 2010;24:239–43. [DOI] [PubMed] [Google Scholar]
- [11].Dong M, Mu N, Guo F, et al. The beneficial effects of postconditioning on no-reflow phenomenon after percutaneous coronary intervention in patients with ST-elevation acute myocardial infarction. J Thromb Thrombolysis 2014;38:208–14. [DOI] [PubMed] [Google Scholar]
- [12].Grygier M, Araszkiewicz A, Lesiak M, et al. Intracoronary adenosine administered during aortocoronary vein graft interventions may reduce the incidence of no-reflow phenomenon. A pilot randomised trial. Kardiol Pol 2014;72:126–33. [DOI] [PubMed] [Google Scholar]
- [13].Rezkalla SH, Kloner RA. No-reflow phenomenon. Circulation 2002;105:656–62. [DOI] [PubMed] [Google Scholar]
- [14].Xu Y, Huo Y, Toufektsian MC, et al. Activated platelets contribute importantly to myocardial reperfusion injury. Am J Physiol Heart Circ Physiol 2006;290:H692–9. [DOI] [PubMed] [Google Scholar]
- [15].Bainey KR, Armstrong PW. Clinical perspectives on reperfusion injury in acute myocardial infarction. Am Heart J 2014;167:637–45. [DOI] [PubMed] [Google Scholar]
- [16].Erer D, Ozer A, Demirtas H, et al. Effects of alprostadil and iloprost on renal, lung, and skeletal muscle injury following hindlimb ischemia-reperfusion injury in rats. Drug Des Devel Ther 2016;10:2651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gezginci-Oktayoglu S, Orhan N, Bolkent S. Prostaglandin-E1 has a protective effect on renal ischemia/reperfusion-induced oxidative stress and inflammation mediated gastric damage in rats. Int Immunopharmacol 2016;36:142–50. [DOI] [PubMed] [Google Scholar]
- [18].Blogowski W, Dolegowska B, Pikula E, et al. The effect of PGE administration on the activity of oxidative system in erythrocytes and platelets during ischemia reperfusion injury and on postoperative renal function in patients undergoing open abdominal aortic aneurysm reconstruction. J Biol Regul Homeost Agents 2012;26:429–38. [PubMed] [Google Scholar]
- [19].Sako H, Hadama T, Miyamoto S, et al. Effect of prostaglandin E1 on ischemia-reperfusion injury during abdominal aortic aneurysm surgery. Surg Today 2006;36:140–6. [DOI] [PubMed] [Google Scholar]
- [20].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Higgins J, Altman D, Gotzsche P. Cochrane handbook for systematic reviews of interventions. BMJ 2008;5:64–74. [Google Scholar]
- [22].Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 2006;25:3443–57. [DOI] [PubMed] [Google Scholar]
- [23].Kleiman NS, Tracy RP, Schaaff LJ, et al. Prostaglandin E1 does not accelerate rTPA-induced thrombolysis in acute myocardial infarction. Am Heart J 1994;127(4 pt 1):738–43. [DOI] [PubMed] [Google Scholar]
- [24].Wu JY, Zhang QY, Wang LY, et al. Study on pharmacological action of prostaglandin E1 on myocardial ischemia-reperfusion injury. Chinese J Clin Pharmacol 1999;15:251. [Google Scholar]
- [25].Fan Y, Jiang Y, Fu X, et al. Effects of liposomal prostaglandin E1 on periprocedural myocardial injury in patients with unstable angina undergoing an elective percutaneous coronary intervention. Coron Artery Dis 2015;26:671–7. [DOI] [PubMed] [Google Scholar]
- [26].Luo CF, Wu X, Hu X. Protective effect of lipid microspheres 1 on myocardial injury following elective percutaneous coronary intervention in patients with angina pectoris: a pilot study. J Cardiovasc Med (Hagerstown) 2011;12:790–4. [DOI] [PubMed] [Google Scholar]
- [27].Shechter M, Agranat O, Har-Zahav Y, et al. Prostaglandin E1 during angioplasty as preventative therapy for coronary restenosis. Am J Ther 1997;4:395–400. [DOI] [PubMed] [Google Scholar]
- [28].Wei LY, Fu XH, Li W, et al. Effect of intravenous administration of liposomal prostaglandin E1 on microcirculation in patients with ST elevation myocardial infarction undergoing primary percutaneous intervention. Chin Med J (Engl) 2015;128:1147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].He ZF, Li ZJ. Prostaglandin E1 reduces reperfusion injury of myocardium by inhibiting cytokines production during cardiac surgery. Zhejiang Da Xue Xue Bao Yi Xue Ban 2004;33:353–6. [DOI] [PubMed] [Google Scholar]
- [30].Kawamura T, Nara N, Kadosaki M, et al. Prostaglandin E1 reduces myocardial reperfusion injury by inhibiting proinflammatory cytokines production during cardiac surgery. Crit Care Med 2000;28:2201–8. [DOI] [PubMed] [Google Scholar]
- [31].Yang YF, Peng K, Liu H, et al. Dexmedetomidine preconditioning for myocardial protection in ischemia-reperfusion injury in rats by downregulation of the HMGB1-TLR4-NF-kappaB signaling pathway. Clin Exp Pharmacol Physiol 2016;44:353–61. [DOI] [PubMed] [Google Scholar]
- [32].Che N, Ma Y, Xin Y. Protective role of fucoidan in cerebral ischemia-reperfusion injury through inhibition of MAPK signaling pathway. Biomol Ther (Seoul) 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Luo LN, Xie Q, Zhang XG, et al. Osthole decreases renal ischemia-reperfusion injury by suppressing JAK2/STAT3 signaling activation. Exp Ther Med 2016;12:2009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Badalzadeh R, Azimi A, Alihemmati A, et al. Chronic type-I diabetes could not impede the anti-inflammatory and anti-apoptotic effects of combined postconditioning with ischemia and cyclosporine A in myocardial reperfusion injury. J Physiol Biochem 2016;73:111–20. [DOI] [PubMed] [Google Scholar]
- [35].DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007;28:105–14. [DOI] [PubMed] [Google Scholar]


