Abstract
Rationale:
Retroperitoneal metastatic lymph node is rare but severe, which has important structures like the gastrointestinal tract and large blood vessels around and may challenge excision, inducing serious complications like hemorrhage, intestinal adhesion, and even death after injury.
Patient concerns:
We described the case of a 60-year-old man with a history of right liver resection in 2010, pulmonary wedge resection in 2012, and transarterial chemoembolization twice in 2014, in which the postoperative pathology suggested the mixed liver cancer, and poorly differentiated lung cancer from liver metastasis.
Diagnoses:
Preoperative magnetic resonance (MR) imaging scan showed a refractory retroperitoneal metastatic lymph node.
Interventions:
Then this patient repeatedly received 4 ablations with US-guided laser ablation within a month.
Outcomes:
After 4 ablations due to residual tumor, MR, and CT images of 5-month follow-up showed the partial response. No obvious side effects were discovered in this case during these procedures.
Lessons:
This suggested US-guided laser ablation appears to be a useful technique for retroperitoneal metastatic lymph node with poor general condition or those refusing surgical therapy.
Keywords: ablation, laser ablation, lymph node, retroperitoneal tumor, ultrasound
1. Introduction
Retroperitoneal lymph nodes metastasis is rare but severe, which means advanced stage or terminal stage of malignancy.[1–5] Recently, many ablation therapies for abdominal metastatic tumors were used such as radiofrequency ablation (RFA),[6,7] microwave ablation (MWA),[8,9] cryoablation,[10] irreversible electroporation (IRE),[11] and high-intensity focused ultrasound (HIFU).[12,13] Retroperitoneal deep tumors from hepatic, colorectal, renal, prostate, and ovarian carcinomas have important structures like the gastrointestinal tract and large blood vessels around, which may challenge excision, and easily induce serious complications like hemorrhage, intestinal adhesion, and even death after injury. Chemotherapy or radiotherapy could be used but sometimes the tumors are not susceptible to these treatments, in which patients may not accept the procedures. Recently, EUS-guided Nd:YAG laser ablation was conducted for recurrent pancreatic neuroendocrine tumor at 4 W for 300 seconds.[14] One year after laser ablation, the coagulative necrosis was still remained. There were emerging animal studies focused on laser ablation for pancreas.[15–17] In swine model, laser ablation was used for normal pancreatic tissue, with an output power of 2 and 3 W and a total delivered energy of 500 and 1000 J.[18] The ablation area was between 49 and 80 mm2 without major postoperative complications. Rather than 14- to 17-gauge needle electrode used in RFA and MWA, laser ablation has thinner 21-gauge puncture needle, which minimized surrounding normal tissue damage.
Here, we describe the case of retroperitoneal metastatic lymph node ablated with ultrasound (US)-guided Nd:YAG laser, whose lesions regressed after 4 repeated ablations.
2. Case report
A 60-year-old man presented with a retroperitoneal metastatic lymph node in magnetic resonance (MR) imaging with a history of right liver resection in 2010, pulmonary wedge resection in 2012, and transarterial chemoembolization twice in 2014, in which the postoperative pathology suggested the mixed liver cancer, and poorly differentiated lung cancer from liver metastasis. At examination, the tumor was very close to the duodenum, pancreas, stomach, and hepatic portal vein. The patient with unresectable masses had hypertension over 10 years and chronic HBV-related cirrhosis for 30 years when he was treated with lamivudine 100 mg qd, and adefovir dipivoxil 10 mg qd for 6 years. He kept a constant body temperature of 37 °C, the blood pressure of 148/96 mm Hg, and the pulse of 77 beats every minute. The patient had no obvious sense of abdominal pain, abdominal distention, nausea, and vomiting. On a test of tumor markers levels, the results were normal, which showed carcinoembryonic antigen 3.7 ng/mL, alpha fetoprotein 17.3 ng/mL, and carbohydrate antigen 19–9 levels 6.1 U/mL. In this study, the procedure was approved by the ethics committee of the First Affiliated Hospital of Zhejiang University. The efficacy of local ablation was estimated with Choi criteria, which appraised the variations both in tumor size and lesion density on computed tomography (CT) imaging: complete response, disappearance of all lesions and no new lesions; partial response (PR), a decrease in size of 10% or a decrease in tumor density over 15% on CT and no new lesions; stable disease, not fit for complete response, PR, or progressive disease; and progressive disease, an increase in tumor size over 10% and not fit for PR by tumor density on CT or new lesions.[19]
Preoperative MR imaging scan showed a mass of 2.8 × 3.5 cm in size close to the duodenum, pancreas, and blood vessels (Fig. 1A). During substance phase, MR images indicated mildly high signal intensity around the tumor and intermediate low signal intensity in the solid component and in the walls (Fig. 1B). Before the initial ablation, there was a retroperitoneal mild hyperechoic area on axial US image (Fig. 1C). In this study, the inpatient underwent percutaneous transhepatic biopsies and ablations to avoid the nearby blood vessels, the procedure of which was performed about 40 minutes under local anesthesia and mild sedation. We used ultrasonography to real-timely guide the inserting of 2 Nd:YAG laser fibers (Echolaser X4, ESAOTE, Italy) in 0.3 mm diameter through a 21-gauge Chiba needle into the lesions (Fig. 1D). Although part of the heat ran off by blood flow,[20] ablation could keep valid area from 12 to 15 mm with the power of 5 W in 5 to 6 minutes. Subsequently, immediate ultrasonography showed that the whole lesion was covered with hyperechoic zone (Fig. 1E and F). However, the next day contrast-enhanced ultrasound (CEUS) revealed an abnormal residual of proximal part of the pancreatic head (Fig. 2A). At 5-day follow-up contrast-enhanced CT images, the lower mass had low signal intensity and upper tumor revealed intermediate high signal intensity of residual in substance phase (Fig. 2B and C). Surprizingly, 1-week follow-up CEUS image showed an enlarged retroperitoneal lymph node located near the pancreatic head, which could be fusion of these residuary small nodules (Fig. 2D). Then the patient undergoing the 2nd ablation with 2 laser fibers showed the lesions well-defined hyperechoic zone (Fig. 2E and F). On the 2nd day, postoperative CEUS images showed they were still remanent (Fig. 2G and I). At the corresponding MR imaging, it also showed this in left of tumor (Fig. 2H). Thus, the 3rd laser ablation was performed (Fig. 2J) and 3 days later CEUS found that there was still a minor lesion located in the separation gap of last 2 needles (Fig. 3A). Subsequently the patient had the 4th ablation along this separation gap until hyperechoic overlay (Fig. 3B–D), then 3 days later, based on these 4 treatments, enhanced CT image of the retroperitoneal mass suggested complete necrosis (Fig. 3E–G). After the following 1 month, substance phase MR revealed low-intensity signal of tumor necrosis with resolution of his problems (Fig. 3H). Until 5 months after 4 ablations, CT showed the tumor PR with little enhanced recurrence (yellow arrows), which located in the left lower edge of original lesions (Fig. 4A and B). On laboratory test of tumor markers, the levels of carcinoembryonic antigen, alpha fetoprotein, and carbohydrate antigen 19–9 indicated 3.6 ng/mL, 26.7 ng/mL, and 8.1 U/mL, respectively. No obvious side effects were discovered in this case during these procedures.
Figure 1.
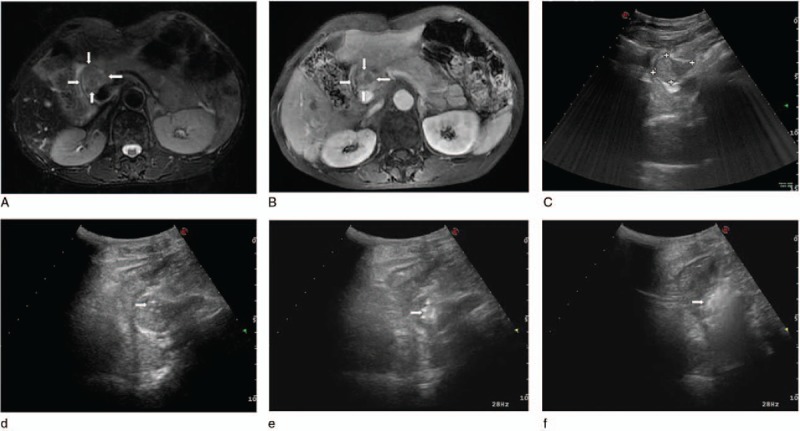
(A) Preoperative axial T2-weighted (white arrows), (B) substance phase MRI image of the abdomen showed a mass adjacent to the hepatic portal vein, pancreas, and stomach (white arrows). (C) Axial gray-scale US image of the retroperitoneal region showed the mild hyperechoic area. (D) Intraoperative sonogram indicated the arranging needle method of using 2 laser fibers parallelly ablating the tumor under US guidance. Initial postoperative immediate US showed local enhancement (arrowhead) (E) and global enhancement (arrowhead) (F) of the lesions. MRI = magnetic resonance imaging, US = ultrasound.
Figure 2.
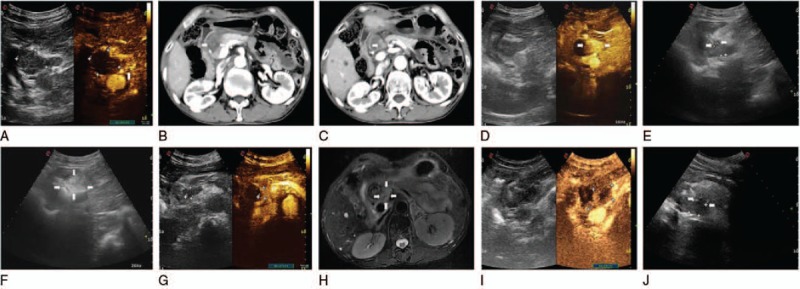
(A) Comparing to conventional ultrasound, the CEUS of the next day showed a little remanent tumor (arrowhead), and there was no detectable enhancement in the necrosis area in the center of the whole mass. Coronal contrast-enhanced CT images acquired 5 days after initial ablation showed lower tumor low signal intensity (arrowhead) (B) and upper mass intermediate high signal intensity (arrowhead) (C) in substance phase, and blood vessel was not injured. (D) One week later, these residuary small nodules appear fusion during CEUS image (arrowhead). (E) Two laser fibers were parallelly accurately inserted into the lesion with US guidance (arrowheads). (F) Axial gray-scale US image showed increased echogenicity covering the whole mass (white arrows). On the next day of the 2nd ablation, postoperative CEUS images showed they were still remanent (G). At the corresponding MRI, it also showed this in left of tumor (arrowheads) (H). After the CEUS positioning the remanent tumor (I), the 3rd laser ablation was performed using 2 laser fibers (arrowheads) (J). CEUS = contrast-enhanced ultrasound, CT = computed tomography, MRI = magnetic resonance imaging, US = ultrasound.
Figure 3.
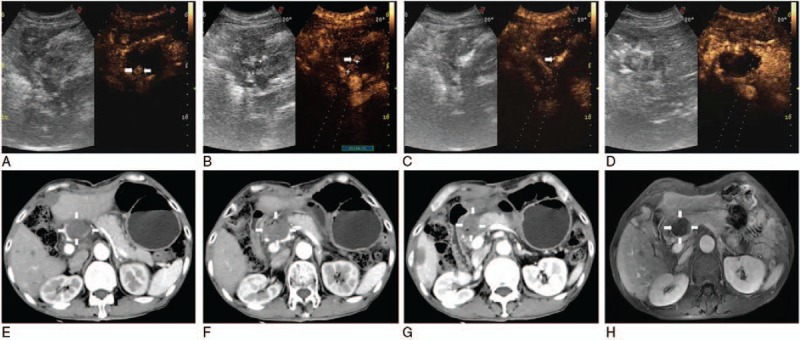
Because remanent tumor was detectable under CEUS guidance 3 days after 3 ablations (arrowheads) (A), the single needle was accurately inserted into the residual lesion (arrowheads) (B), showing local enhancement (arrowheads) (C) and no contrast agents filled (D). Substance phase of CT obtained 3 days after US-guided percutaneous LA showed complete necrosis of the tumor (white arrows) (including upper [white arrows] (E), mid [white arrows] (F), and lower tumor [white arrows] (G) of it), and 1 month later substance phase MR image had corresponding results (H). CEUS = contrast-enhanced ultrasound, CT = computed tomography, LA = laser ablation, MR = magnetic resonance, US = ultrasound.
Figure 4.
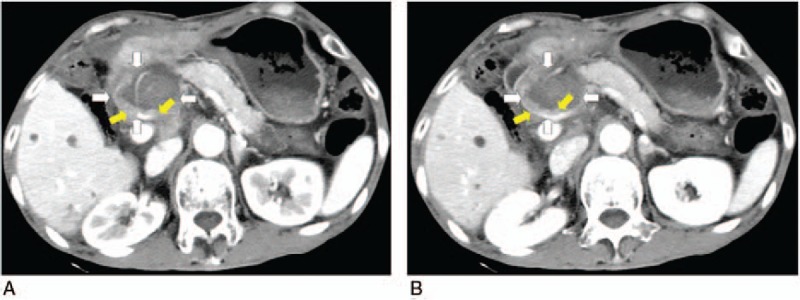
Coronal contrast-enhanced computed tomography (CT) scan obtained 5 months after 4 ablations showed the tumor partial response with little enhanced recurrence (yellow arrows), which located in the left lower edge of original lesions (white arrows) (A, B).
3. Discussion
Retroperitoneal metastatic tumors are rare malignancies, which is usually located deeply near the risky area of bowels, stomach, liver, or pancreas. In our center, about 950 cases of patients with malignant tumor underwent RFA and MWA each year. In this case, the lesions were PR by laser ablation based on US guidance. Comparing with other thermal ablations like percutaneous CT- or US-guided RFA, cryotherapy of the retroperitoneal metastatic lymph nodes from hepatocellular carcinoma, retroperitoneal schwannoma, soft-tissue tumors, and liposarcoma,[21–25] in which the common postoperative complications included hemorrhage, skin burn, hematoma, and pain after treatment. Laser ablation has thinner puncture needle than needle electrode used in RFA and MWA, more precise thermal effect and effective range than other thermal ablations. Furthermore, because CT image was based on cross-sectional anatomy,[26] it would be forbidden if the lymph node is masked by some organs like the bowel. However, under real-time US guidance, the needle could freely advance into the center of deep position, which was feasible to navigate the needle away from the important structures. Moreover, there was no still report of needle implantation metastases in PLA. Although there are residual tumors, due to these advantages, it might enable PLA eligible for repeated ablations. However, major limitations of this study were limited sample size and short follow-up period. In the future, we will further increase the sample size and follow-up period to observe the curative effect of the treatments.
This study showed US-guided percutaneous laser ablation is feasible, minimally invasive and effective, which may be a potential candidate of retroperitoneal metastatic lymph node with poor general condition or those refusing surgical therapy.
Acknowledgments
The authors thank the Transverse Project Foundation of Zhejiang University (K16-518051 086) for the support.
Footnotes
Abbreviations: CEUS = contrast-enhanced ultrasound, CT = computed tomography, MR = magnetic resonance, MWA = microwave ablation, PR = partial response, RFA = radiofrequency ablation, US = ultrasound.
Authorship: GT, TJ: acquisition of data, critical revision of the manuscript for important intellectual content, technical, or material support; TJ: study concept and design, obtained funding, and study supervision; and GT: analysis and interpretation of data, drafting of the manuscript, statistical analysis.
Funding/support: This study was supported by the Transverse Project Foundation of Zhejiang University (K16-518051-086).
The authors have no conflicts of interest to disclose.
References
- [1].Hino H, Kagawa H, Kinugasa Y, et al. Long-term survival with surgery for metachronous retroperitoneal lymph node and pancreatic metastases after curative resection of rectal cancer: a case report. Surg Case Rep 2016;2:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Keyver-Paik MD, Arden JM, Luders C, et al. Impact of chemotherapy on retroperitoneal lymph nodes in ovarian cancer. Anticancer Res 2016;36:1815–24. [PubMed] [Google Scholar]
- [3].Stepanian S, Patel M, Porter J. Robot-assisted laparoscopic retroperitoneal lymph node dissection for testicular cancer: evolution of the technique. Eur Urol 2016;70:661–7. [DOI] [PubMed] [Google Scholar]
- [4].Atri M, Zhang Z, Dehdashti F, et al. Utility of PET-CT to evaluate retroperitoneal lymph node metastasis in advanced cervical cancer: results of ACRIN6671/GOG0233 trial. Gynecol Oncol 2016;142:413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pearce SM, Golan S, Gorin MA, et al. Safety and early oncologic effectiveness of primary robotic retroperitoneal lymph node dissection for nonseminomatous germ cell testicular cancer. Eur Urol 2016;71:476–82. [DOI] [PubMed] [Google Scholar]
- [6].Kelly EF, Leveillee RJ. Image guided radiofrequency ablation for small renal masses. Int J Surg 2016;36:525–32. [DOI] [PubMed] [Google Scholar]
- [7].Varghese M, Bruland O, Wiedswang AM, et al. Metastatic mesenteric dedifferentiated leiomyosarcoma: a case report and a review of literature. Clin Sarcoma Res 2016;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Qi C, Yu XL, Liang P, et al. Ultrasound-guided microwave ablation for abdominal wall metastatic tumors: a preliminary study. World J Gastroenterol 2012;18:3008–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Brace CL, Hinshaw JL, Lubner MG. Thermal ablation for the treatment of abdominal tumors. J Visual Exp 2011;7:2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liang Z, Fei Y, Lizhi N, et al. Percutaneous cryotherapy for metastatic bladder cancer: experience with 23 patients. Cryobiology 2014;68:79–83. [DOI] [PubMed] [Google Scholar]
- [11].Marsanic P, Mellano A, Sottile A, et al. Irreversible electroporation as treatment of locally advanced and as margin accentuation in borderline resectable pancreatic adenocarcinoma. Med Biol Eng Comput 2017. [DOI] [PubMed] [Google Scholar]
- [12].Zhang M, Liu L, Wang J, et al. Effects of high-intensity focused ultrasound for treatment of abdominal lymph node metastasis from gastric cancer. J Ultrasound Med 2015;34:435–40. [DOI] [PubMed] [Google Scholar]
- [13].Ritchie RW, Leslie TA, Turner GD, et al. Laparoscopic high-intensity focused ultrasound for renal tumours: a proof of concept study. BJU Int 2011;107:1290–6. [DOI] [PubMed] [Google Scholar]
- [14].Di MF, Picconi F, Martino M, et al. Endoscopic ultrasound-guided Nd:YAG laser ablation of recurrent pancreatic neuroendocrine tumor: a promising revolution? Endoscopy 2014;46:E380–1. [DOI] [PubMed] [Google Scholar]
- [15].Allegretti G, Saccomandi P, Giurazza F, et al. Magnetic resonance-based thermometry during laser ablation on ex-vivo swine pancreas and liver. Med Eng Phys 2015;37:631–41. [DOI] [PubMed] [Google Scholar]
- [16].Schena E, Majocchi L. Assessment of temperature measurement error and its correction during Nd:YAG laser ablation in porcine pancreas. Int J Hyperthermia 2014;30:328–34. [DOI] [PubMed] [Google Scholar]
- [17].Schena E, Saccomandi P, Giurazza F, et al. Experimental assessment of CT-based thermometry during laser ablation of porcine pancreas. Phys Med Biol 2013;58:5705. [DOI] [PubMed] [Google Scholar]
- [18].Di MF, Martino M, Rea R, et al. EUS-guided Nd:YAG laser ablation of normal pancreatic tissue: a pilot study in a pig model. Gastrointest Endosc 2010;72:358. [DOI] [PubMed] [Google Scholar]
- [19].Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 2007;25:1753–9. [DOI] [PubMed] [Google Scholar]
- [20].Frericks BB, Ritz JP, Albrecht T, et al. Influence of intrahepatic vessels on volume and shape of percutaneous thermal ablation zones: in vivo evaluation in a porcine model. Invest Radiol 2008;43:211–8. [DOI] [PubMed] [Google Scholar]
- [21].Machi J, Oishi AJ, Furumoto NL, et al. Sonographically guided radio frequency thermal ablation for unresectable recurrent tumors in the retroperitoneum and the pelvis. J Ultrasound Med 2003;22:507–13. [DOI] [PubMed] [Google Scholar]
- [22].Keil S, Bruners P, Brehmer B, et al. Percutaneous radiofrequency ablation for treatment of recurrent retroperitoneal liposarcoma. Cardiovasc Interv Radiol 2008;31(Suppl 2):S213–6. [DOI] [PubMed] [Google Scholar]
- [23].Gao F, Gu Y, Huang J, et al. Radiofrequency ablation of retroperitoneal metastatic lymph nodes from hepatocellular carcinoma. Acad Radiol 2012;19:1035–40. [DOI] [PubMed] [Google Scholar]
- [24].Zhao M, Li X, Wang J, et al. Retroperitoneal schwannoma treated with percutaneous computed tomography-guided radiofrequency ablation. J Neurosurg Spine 2012;17:173–6. [DOI] [PubMed] [Google Scholar]
- [25].Littrup PJ, Bang HJ, Currier BP, et al. Soft-tissue cryoablation in diffuse locations: feasibility and intermediate term outcomes. J Vasc Interv Radiol 2013;24:1817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hof I, Arbab-Zadeh A, Scherr D, et al. Correlation of left atrial diameter by echocardiography and left atrial volume by computed tomography. J Cardiovasc Electrophysiol 2009;20:159–63. [DOI] [PubMed] [Google Scholar]


