Figure 3.
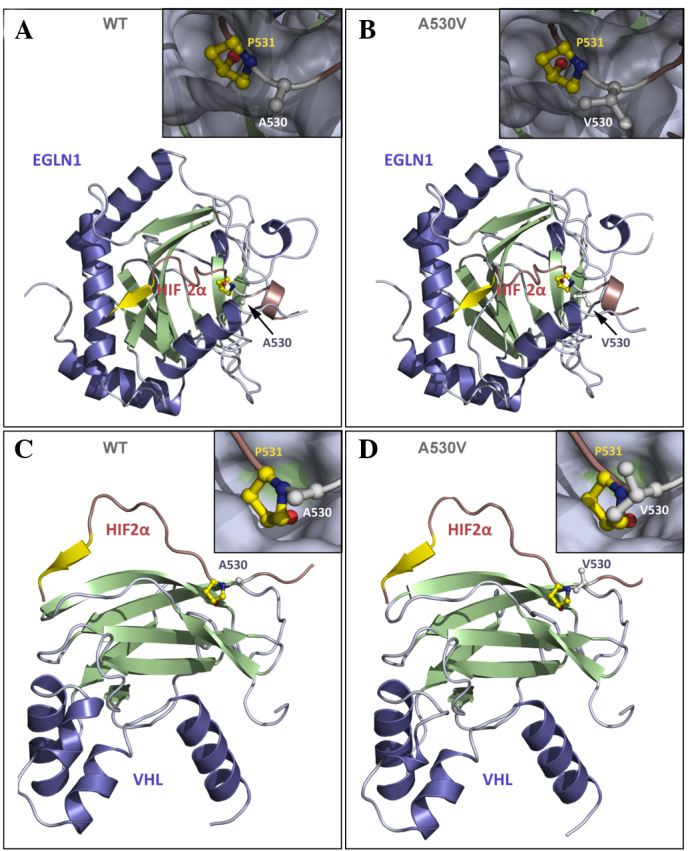
Representation of human HIF2α in the presence of its binding partners EGLN1 and VHL. (A) WT HIF2α (A560) interacting with EGLN1; (B) mutant Val560 interacting with EGLN1; (C) WT HIF2α interacting with VHL; and (D) mutant Val560 interacting with VHL. HIF2α (16 residues) is represented in red and yellow, and the interactive partners (EGLN1 or VHL) are represented in blue and green. (B and D) The inserts present a closer view of Pro531 and Ala530 (or Val530) from HIF2α in the ball-and-stick representation to show the atomic details, while HIF-2α partners, (A and B) EGLN1 or (C and D) VHL are shown as a grey surface showing that residues 530 and 531 bind to small pockets at the surface of the protein partner. Residue Ala530 is located in close proximity to residue Pro531, which is hydroxylated by EGLN1 and at the interface with the binding partners EGLN1 and VHL. Hydroxylation of Pro531 is required for interaction with VHL. It is anticipated that valine, which is a larger residue than alanine, increases steric hindrance at Pro531, resulting in a reduction in its accessibility to EGLN1 by inhibition of Pro531 hydroxylation; therefore, interaction with VHL and subsequent ubiquitination is prevented. Panel D is presented as a model, but VHL interaction should not occur in the Val530 mutant. HIF2α, hypoxia inducible factor 2α; EGLN1, Egl-9 family hypoxia-inducible factor 1; VHL, von Hippel-Lindau tumor suppressor; WT, wild-type.
