Abstract
It has been demonstrated that various long non-coding RNAs (lncRNAs) may have key roles in various types of cancer. Clear cell renal cell carcinoma (ccRCC) is the most common subtype of all RCCs, accounting for 70–80% of all cases. The present study identified a novel lncRNA and investigated its clinical significance and physiological function in ccRCC. The expression pattern of the novel lncRNA LOC389332 in 30 ccRCC tissue samples was examined using reverse-transcription quantitative polymerase chain reaction. The results demonstrated that LOC389332 expression was markedly lower in ccRCC tissues compared with that in matched adjacent non-tumor tissues. Of note, downregulation of LOC389332 expression was significantly associated with the tumor American Joint Commission on Cancer stage (P=0.001), Fuhrman grade (P=0.001) and lymph node metastasis (P<0.001). Furthermore, patients with ccRCC with lower levels of LOC389332 expression had a shorter overall survival time than those with higher LOC389332 expression. A gain-of-function study was used to evaluate the biological function of LOC389332 in ccRCC and the results suggested that restoration of LOC389332 expression inhibited the growth and migration of the 786-O and 769-P cell lines. Therefore, the results of the present study demonstrated that LOC389332 is a novel lncRNA involved in ccRCC progression and may be a potential diagnostic and prognostic biomarker. Ectopic overexpression of LOC389332 may represent a therapeutic strategy for ccRCC.
Keywords: clear cell renal cell carcinoma, LOC389332, downregulation, prognostic biomarker, progression
Introduction
Renal cell carcinoma (RCC) has the highest mortality rate of all urological malignancies worldwide and accounts for ~65,000 new cancer cases per year in the United States alone (1). Clear cell (cc) RCC is the most common subtype and accounts for 70–80% of all RCC cases (2,3). Metastasis and progression are the most common events in ccRCC and ~33% of affected patients are in the terminal stage at the time of diagnosis (4,5). Patients with metastatic ccRCC have a poor prognosis and the number of therapeutic strategies available is limited (6–8). Therefore, further research into the underlying molecular mechanisms of ccRCC metastasis to identify novel diagnostic biomarkers for ccRCC in the early stages is urgently required.
Long non-coding RNAs (lncRNAs) are a newly identified class of non-coding RNAs, with transcripts of >200-bp nucleotides that have no protein-coding function (9). It has been demonstrated that lncRNAs may serve a crucial role in various cellular biological processes and human disease (10,11). As microRNAs, lncRNAs may serve a key role in regulating human cancer cell growth, invasion and apoptosis (12,13). Li et al (14) demonstrated that the upregulation of lncRNA urothelial cancer associated (UCA) 1 was correlated with advanced clinical stage and a poor prognosis in patients with esophageal squamous cell carcinoma. In addition, knockdown of UCA1 was observed to inhibit cell growth, migration and invasion (14). The lncRNA HOXA cluster antisense RNA 2 promotes cancer cell proliferation by epigenetically silencing P21/Polo-like kinase 3/DNA damage inducible transcript 3 expression in gastric carcinoma (15). Furthermore, the lncRNA taurine upregulated gene 1 is highly expressed in hepatocellular carcinoma and promotes cell proliferation and apoptosis by epigenetically silencing Krüppel-like factor 2 (16).
LOC389332 is a 723-bp intragenic lncRNA transcribed from chromosome 5 in the human genome (17). Analysis of previous lncRNA expression signatures by microarray showed that LOC389332 was significantly downregulated in ccRCC (18,19); however, its biological functions in ccRCC have remained elusive. The present study was performed to verify the expression pattern of LOC389332 and evaluate the clinical significance of lncRNA LOC389332 in ccRCC. In addition, the impact of LOC389332 on ccRCC cell proliferation and migration was assessed in vitro. The present study aimed to identify whether LOC389332 downregulation was associated with poor prognosis and tumor progression in ccRCC, therefore determining whether LOC389332 may be developed as a novel prognostic biomarker and therapeutic target for ccRCC treatment.
Materials and methods
Patients and specimens
All 30 samples of ccRCC tissues and paired adjacent non-tumor tissues used in the present study were obtained from 30 patients who had undergone radical nephrectomy at the Department of Urology of Renmin Hospital, Hubei University of Medicine (Hubei, China) between January 2009 and February 2011. Patients were selected on the basis of the following inclusion and exclusion criteria: i) Definite pathological diagnosis of ccRCC according to the World Health Organization criteria (20); ii) suitable formalin-fixed, paraffin-embedded tissue specimens; iii) complete clinicopathological data. None of the patients received radiotherapy or chemotherapy prior to radical nephrectomy. Following surgical resection, specimens were immediately immersed in RNAlater® (Qiagen GmbH, Hilden, Germany) for 30 min and then transferred into liquid nitrogen for cryopreservation until use. The clinicopathological features of the patients are presented in Table I. Follow-up of patients was completed in May 2015 and the median observation time was 30 months. All specimens were collected on the basis of their availability for research and following a protocol approved by the Medical Ethics committee of the Renmin Hospital, Hubei University of Medicine (Hubei, China). Written consent was obtained from all patients participating in the study.
Table I.
Association between LOC389332 expression and clinicopathological parameters of patients with ccRCC.
| LOC389332 expression | ||||
|---|---|---|---|---|
| Clinicopathological parameter | Number of cases | Low (n) | High (n) | P-value |
| Gender | 1.000 | |||
| Male | 15 | 11 | 4 | |
| Female | 15 | 10 | 5 | |
| Age, years | 0.418 | |||
| ≤60 | 18 | 14 | 4 | |
| >60 | 12 | 7 | 5 | |
| Tumor size, cm | 0.100 | |||
| ≤4 | 19 | 11 | 8 | |
| >4 | 11 | 10 | 1 | |
| Fuhrman grade | 0.001 | |||
| T1-T2 | 7 | 1 | 6 | |
| T3-T4 | 23 | 20 | 3 | |
| AJCC stage | 0.001 | |||
| I–II | 9 | 2 | 7 | |
| III–IV | 21 | 19 | 2 | |
| Lymph node metastasis | <0.001 | |||
| Absent | 10 | 1 | 9 | |
| Present | 20 | 20 | 0 | |
AJCC stage, tumor stage according to the American Joint Commission on Cancer.
Cell culture
Human ccRCC cell lines (786-O, 769-P, CaKi-1 and RLC-310) and a normal immortalized human proximal tubule epithelial cell line (HK-2) were obtained from the American Type Culture Collection (Manassas, VA, USA). All cells were cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA), supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) in a 100% humidified atmosphere of 5% CO2/95% air at 37°C.
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from 30 cases of fresh ccRCC tissues and cell lines were extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RNA was detected and quantified using a NanoDrop™ 2000c spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE, USA). Subsequently, RNA samples were reverse-transcribed into complementary first-strand DNA using a High-Capacity RNA-to-cDNA kit (Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. The qPCR reaction was then performed on the LightCycler® 480 Real-Time PCR system (Roche Applied Science, Penzberg, Germany) using the SYBR® Select Master Mix (Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. The reaction conditions for PCR were as follows: 95°C for 20 min, followed by 40 cycles of 95°C for 10 sec, 55°C for 30 sec and 72°C for 30 sec. The specific primers for human LOC389332 were as follows: Forward, 5′-GCGCTCGTCGTCCTCTTCATCG-3′ and reverse, 5′-CGTGGCTCAGTCCCAAGCTACACC-3′. The GAPDH gene was used as an internal control, and the primers for GAPDH were: Forward, 5′-GGCACCACACCTTCTACAATGAG-3′ and reverse, 5′-GGATAGCACAGCCTGGATAGCA-3′. All primers were obtained from Invitrogen (Carlsbad, CA, USA). The relative expression levels of LOC389332 in ccRCC tissues and cell lines were analyzed using the 2−ΔΔCq method (21).
Cell transfection
As reduction in the expression of LOC389332 in ccRCC tissues and cells was observed, a gain-of-function study was performed by inducing the overexpression of LOC389332 to identify its function in the 786-O and 769-P cell lines. An LOC389332 overexpression plasmid (pcDNA3.1-LOC389332) was purchased from Thermo Fisher Scientific, Inc. 786-O and 769-P cells were treated with the indicated amounts of pcDNA3.1-LOC389332 plasmid using Lipofectamine® 2000 reagent (Thermo Fisher Scientific, Inc.). The empty vector pcDNA3.1- was used as a negative control.
Cell migration assay
A scratch wound assay was applied to evaluate the ability of 786-O and 769-P cells to migrate in vitro. Cells (~2×106/well) were seeded into 6-well plates and grown to 70% confluence ~24 h prior to infection. Following 6 h of transfection, similar-sized wounds were generated to the cell monolayers using sterile 100-µl pipette tips. Following three washes with phosphate-buffered saline to remove cell debris, cells were cultured in the incubator at 37°C. In each sample, images of the same area were acquired with a Leica DM LB2 microscope digital camera system (Leica Microsystems GmbH, Wetzlar, Germany) at 0 and 12 h after the wounds were made to determine the amount of wound closure. The data was calculated using the software program MIAS-2000 (Leica Microsystems GmbH). This experiment was performed in triplicate and repeated at least three times.
Cell proliferation assay
786-O and 769-P cell proliferation was assessed using an MTT assay. Transfected cells were seeded onto each well of 96-well plates at a density of 5×103 cells per well. Following 0, 24, 48, 72 and 96 h of transfection, 15 µl of MTT (5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) was added to each well and plates were incubated for 4 h at room temperature. Subsequently, 130 µl dimethyl sulfoxide was added to each well and wells were shaken for 10 min at 37°C to solubilize the formazan crystals. The absorbance of 786-O and 769-P cells was measured at 490 nm using a Bio-Rad model 680 microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, USA). Results from the MTT assay are presented as the mean of at least three independent experiments.
Statistical analysis
Statistical analysis was performed with SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). Values are expressed as the mean ± standard deviation. To compare LOC389332 expression levels in ccRCC tissues vs. matched tumor normal tissues, a paired Student's t-test. was used. The χ2 test was applied to compare the levels of LOC389332 expression with various clinicopathological parameters. The results of the MTT assay were analyzed using one-way analysis of variance. Overall survival curves were generated using the Kaplan-Meier method. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of LOC389332 is downregulated in ccRCC tissues and cells
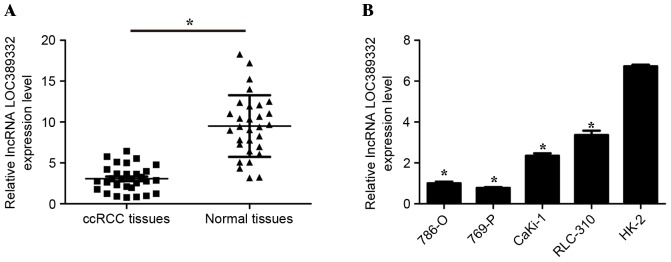
Previous lncRNA expression profiling of ccRCC indicated that LOC389332 was significantly downregulated in RCC tissues (18,19). To confirm that LOC389332 expression is decreased in ccRCC, RT-qPCR was performed to quantify LOC389332 expression in the 30 ccRCC tissues and ccRCC cell lines. The results demonstrated that LOC389332 expression was significantly lower in ccRCC tissues compared with matched adjacent normal tissues (P<0.05; Fig. 1A). In addition, LOC389332 expression was significantly downregulated in 786-O, 769-P, CaKi-1 and RLC-310 cell lines, compared with that in the normal HK-2 cell line (P<0.05; Fig. 1B). These results suggested that LOC389332 expression is suppressed in ccRCC.
Figure 1.
lnRNA LOC389332 expression is downregulated in ccRCC tissues and cells. (A) Relative LOC389332 expression was significantly downregulated in ccRCC tissues compared with matched adjacent normal tissues. The bars through the data points indicate the mean ± standard deviation. (B) Relative LOC389332 expression was significantly lower in ccRCC cell lines than in the normal HK-2 cell line. *P<0.05 vs. HK-2. Data are expressed as the mean ± standard deviation (n=3). lncRNA, long non-coding RNA; ccRCC, clear cell renal cell carcinoma.
Association between LOC389332 expression and clinicopathological parameters in ccRCC
To evaluate the association between LOC389332 expression and the patients' clinicopathological features, the 30 ccRCC samples were divided into low (n=21) and high (n=9) LOC389332 expression groups based on the median value of relative LOC389332 expression. As presented in Table I, downregulation of LOC389332 expression was correlated with tumor American Joint Commission on Cancer (AJCC) stage (22) (P=0.001), Fuhrman grade (23) (P=0.001) and lymph node metastasis (P<0.001). However, no correlation was detected between LOC389332 expression and patient gender (P=1.000), age (P=0.418) or tumor size (P=0.100). These results demonstrated that decreased LOC389332 expression was associated with ccRCC progression and development.
Downregulation of LOC389332 predicts poor prognosis in ccRCC patients
Kaplan-Meier analysis revealed that the overall survival rate of the low LOC389332 expression group was significantly lower than the overall survival of the high LOC389332 expression group (P=0.001; Fig. 2). The 3-year survival rate for ccRCC patients with low LOC389332 expression was 54% compared with 83% in patients with high LOC389332 expression (P<0.05).
Figure 2.
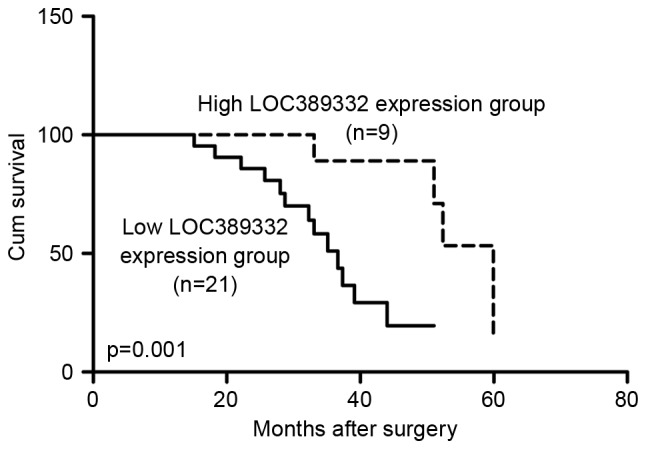
Downregulation of lncRNA LOC389332 predicts poor prognosis in ccRCC patients. Kaplan-Meier survival curves for 30 patients with ccRCC according to high or low levels of LOC389332 expression, defined on the basis of the median value of relative LOC389332 expression. The 3-year survival rate was 72% in the high LOC389332 group (n=9) and 54% in the low LOC389332 group (n=21; P=0.001). Cum survival, cumulative survival; lncRNA, long non-coding RNA; ccRCC, clear renal cell carcinoma.
Downregulation of LOC389332 inhibits growth of ccRCC cells in vitro
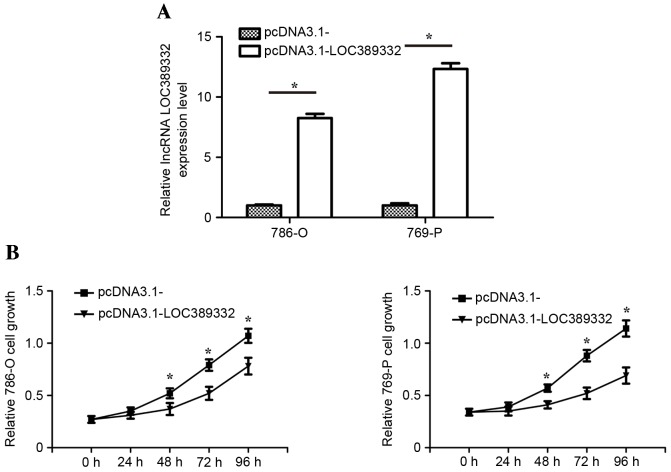
To identify the biological functions of LOC389332 on ccRCC cells in vitro, transfection of pcDNA3.1-LOC389332 overexpression plasmid were applied to restore LOC389332 expression in 786-O and 769-P cells. RT-qPCR analysis revealed that LOC389332 expression in 786-O and 769-P cells transfected with the pcDNA3.1-LOC389332 plasmid was significantly upregulated compared with that in the pcDNA3.1-empty vector group (Fig. 3A). The MTT assay revealed that the proliferation of the 786-O and 769-P cell lines was significantly decreased in the pcDNA3.1-LOC389332 groups compared with that in the empty vector-transfected groups (Fig. 3B).
Figure 3.
Downregulation of lncRNA LOC389332 inhibits growth of ccRCC cells in vitro. (A) LOC389332 expression in 786-O and 769-P cells following transfection with pcDNA3.1-LOC389332 overexpression plasmid was significantly higher than in those treated with the pcDNA3.1- (empty vector). (B) Proliferation of 786-O and 769-P cells was significantly decreased following treatment with pcDNA3.1-LOC389332 plasmid. Each experiment was independently repeated three times. *P<0.05 vs. pcDNA3.1-LOC389332. lncRNA, long non-coding RNA; ccRCC, clear renal cell carcinoma.
Downregulation of LOC389332 inhibits migration of ccRCC cells in vitro
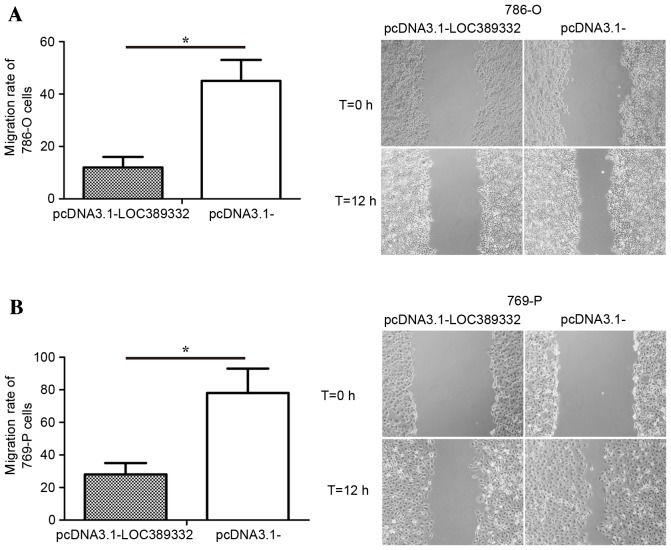
The effect of LOC389332 on ccRCC cell migration was observed using a scratch wound assay. Compared with the pcDNA3.1-empty vector group, overexpression of LOC389332 significantly suppressed the migration capacity of 786-O and 769-P cells (P=0.002 in each; Fig. 4), suggesting that ectopic overexpression of LOC389332 impairs the migratory capacity of ccRCC cells.
Figure 4.
Downregulation of lncRNA LOC389332 inhibits migration of ccRCC cells in vitro. (A) 786-O cells transfected with pcDNA3.1-LOC389332 plasmid or pcDNA3.1-(empty vector), respectively. (B) 769-P cells transfected with pcDNA3.1-LOC389332 plasmid or pcDNA3.1- (empty vector), respectively. All experiments were performed three times and representative images are shown (magnification, ×200). *P<0.05. lncRNA, long non-coding RNA, ccRCC, clear cell renal carcinoma.
Discussion
At present, ccRCC has one of the highest mortality rates of all types of kidney disease (24). In spite of improvements in therapeutic strategies for cancer, major challenges still exist regarding the management of patients with ccRCC. Therefore, identifying novel molecular targets to diagnose and treat ccRCC is important to improve the prognosis and clinical outcomes of patients affected.
Over the past few decades, gene expression microarray analysis has been regarded as a useful and promising approach to identify the molecular signatures of ccRCC (25,26). A number of novel lncRNAs have been identified using lncRNA expression profiling and certain altered lncRNA expression patterns have been found to be associated with the genesis and progression of various types of cancer (27,28). It was demonstrated that high expression of the lncRNA metastasis associated lung adenocarcinoma transcript 1 is correlated with tumor progression and poor prognosis in patients with ccRCC (29). Furthermore, upregulation of lncRNA Sprouty RTK signaling antagonist 4-intronic transcript 1 predicts poor prognosis in patients with ccRCC (30). Downregulation of lncRNA growth arrest specific 5 suppresses tumor growth in ccRCC (31). Furthermore, it was demonstrated that decreased expression of neuroblastoma associated transcript 1 is associated with poor prognosis in patients with ccRCC (32). However, in addition to these few abovementioned lncRNAs, further ones remain to be identified in ccRCC.
Previous studies using lncRNA expression profiling have indicated that lncRNA LOC389332 is significantly downregulated in ccRCC tissue (18,19). However, despite being the most common urological cancer, it remains unknown whether aberrant LOC389332 expression is associated with ccRCC carcinogenesis. The present study investigated the clinical significance of LOC389332 in patients with ccRCC. The RT-qPCR results showed that LOC389332 expression was markedly downregulated in ccRCC tissues and cell lines compared with that in adjacent non-tumor tissues and the normal human proximal tubule epithelial cell line HK-2. In addition, it was demonstrated that LOC389332 expression was strongly associated with the AJCC tumor stage, Fuhrman grade and lymph node metastasis. However, LOC389332 expression was not associated with patient age, gender and tumor size. These results suggested that downregulation of LOC389332 serves an important role in ccRCC progression and development.
The clinicopathological features of patients with ccRCC suggest that LOC389332 may affect the growth and metastasis of ccRCC cells. Therefore, MTT and scratch wound assays were performed to evaluate the biological function of LOC389332 in ccRCC cells. The results demonstrated that ectopic overexpression of LOC389332 significantly inhibits the growth and migratory capacity of 786-O and 769-P cells compared with that of empty vector-transfected cells. This suggested that restoration of LOC389332 expression may inhibit ccRCC development and progression and that high expression of LOC389332 may inhibit malignant phenotypes of ccRCC.
In conclusion, to the best of our knowledge, the present study was the first to identify that the novel lncRNA LOC389332 is downregulated in ccRCC tissues, which is associated with advanced tumor progression. Furthermore, the results suggested that LOC389332 affects ccRCC cell proliferation and migration. Therefore, LOC389332 may be developed as a novel and effective diagnostic and prognostic marker, and ectopic overexpression of LOC389332 may represent a therapeutic strategy for ccRCC.
Acknowledgements
The present study was supported by the Scientific and Technological Project of Shiyan City of Hubei Province (grant no. ZD2012020).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Martel CL, Lara PN. Renal cell carcinoma: Current status and future directions. Crit Rev Oncol Hematol. 2003;45:177–190. doi: 10.1016/S1040-8428(02)00076-8. [DOI] [PubMed] [Google Scholar]
- 3.Rini BI, Rathmell WK, Godley P. Renal cell carcinoma. Curr Opin Oncol. 2008;20:300–306. doi: 10.1097/CCO.0b013e3282f9782b. [DOI] [PubMed] [Google Scholar]
- 4.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 5.Russo P. Renal cell carcinoma: Presentation, staging, and surgical treatment. Semin Oncol. 2000;27:160–176. [PubMed] [Google Scholar]
- 6.Bhatt JR, Finelli A. Landmarks in the diagnosis and treatment of renal cell carcinoma. Nat Rev Urol. 2014;11:517–525. doi: 10.1038/nrurol.2014.194. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 8.Cho IC, Chung J. Current status of targeted therapy for advanced renal cell carcinoma. Korean J Urol. 2012;53:217–228. doi: 10.4111/kju.2012.53.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 11.Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spizzo R, Almeida MI, Colombatti A, Calin GA. Long non-coding RNAs and cancer: A new frontier of translational research? Oncogene. 2012;31:4577–4587. doi: 10.1038/onc.2011.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li JY, Ma X, Zhang CB. Overexpression of long non-coding RNA UCA1 predicts a poor prognosis in patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:7938–7944. [PMC free article] [PubMed] [Google Scholar]
- 15.Xie M, Sun M, Zhu YN, Xia R, Liu YW, Ding J, Ma HW, He XZ, Zhang ZH, Liu ZJ, et al. Long noncoding RNA HOXA-AS2 promotes gastric cancer proliferation by epigenetically silencing P21/PLK3/DDIT3 expression. Oncotarget. 2015;6:33587–33601. doi: 10.18632/oncotarget.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang MD, Chen WM, Qi FZ, Sun M, Xu TP, Ma P, Shu YQ. Long non-coding RNA TUG1 is up-regulated in hepatocellular carcinoma and promotes cell growth and apoptosis by epigenetically silencing of KLF2. Mol Cancer. 2015;14:165. doi: 10.1186/s12943-015-0431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmutz J, Martin J, Terry A, Couronne O, Grimwood J, Lowry S, Gordon LA, Scott D, Xie G, Huang W, et al. The DNA sequence and comparative analysis of human chromosome 5. Nature. 2004;431:268–274. doi: 10.1038/nature02919. [DOI] [PubMed] [Google Scholar]
- 18.Yu G, Yao W, Wang J, Ma X, Xiao W, Li H, Xia D, Yang Y, Deng K, Xiao H, et al. LncRNAs expression signatures of renal clear cell carcinoma revealed by microarray. PLoS One. 2012;7:e42377. doi: 10.1371/journal.pone.0042377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin C, Han Z, Qian J, Bao M, Li P, Ju X, Zhang S, Zhang L, Li S, Cao Q, et al. Expression pattern of long non-coding RNAs in renal cell carcinoma revealed by microarray. PLoS One. 2014;9:e99372. doi: 10.1371/journal.pone.0099372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006;49:798–805. doi: 10.1016/j.eururo.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 21.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 22.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th. Springer Verlag; New York, NY: 2010. pp. 479–489. [Google Scholar]
- 23.Zimmermann U, Woenckhaus C, Pietschmann S, Junker H, Maile S, Schultz K, Protzel C, Giebel J. Expression of annexin II in conventional renal cell carcinoma is correlated with Fuhrman grade and clinical outcome. Virchows Arch. 2004;445:368–374. doi: 10.1007/s00428-004-1103-4. [DOI] [PubMed] [Google Scholar]
- 24.Chow WH, Devesa SS. Contemporary epidemiology of renal cell cancer. Cancer J. 2008;14:288–301. doi: 10.1097/PPO.0b013e3181867628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Spronsen DJ, de Weijer KJ, Mulders PF, De Mulder PH. Novel treatment strategies in clear-cell metastatic renal cell carcinoma. Anticancer Drugs. 2005;16:709–717. doi: 10.1097/01.cad.0000167901.58877.a3. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi M, Teh BT, Kanayama HO. Elucidation of the molecular signatures of renal cell carcinoma by gene expression profiling. J Med Invest. 2006;53:9–19. doi: 10.2152/jmi.53.9. [DOI] [PubMed] [Google Scholar]
- 27.Maruyama R, Suzuki H. Long noncoding RNA involvement in cancer. BMB Rep. 2012;45:604–611. doi: 10.5483/BMBRep.2012.45.11.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lisitsyn NA, Chernyi AA, Karpov VL, Beresten SF. A role of long noncoding RNAs in carcinogenesis. Mol Biol (Mosk) 2015;49:561–570. doi: 10.1134/S002689331504010X. (In Russian) [DOI] [PubMed] [Google Scholar]
- 29.Zhang HM, Yang FQ, Chen SJ, Che J, Zheng JH. Upregulation of long non-coding RNA MALAT1 correlates with tumor progression and poor prognosis in clear cell renal cell carcinoma. Tumour Biol. 2015;36:2947–2955. doi: 10.1007/s13277-014-2925-6. [DOI] [PubMed] [Google Scholar]
- 30.Zhang HM, Yang FQ, Yan Y, Che JP, Zheng JH. High expression of long non-coding RNA SPRY4-IT1 predicts poor prognosis of clear cell renal cell carcinoma. Int J Clin Exp Pathol. 2014;7:5801–5809. [PMC free article] [PubMed] [Google Scholar]
- 31.Qiao HP, Gao WS, Huo JX, Yang ZS. Long non-coding RNA GAS5 functions as a tumor suppressor in renal cell carcinoma. Asian Pac J Cancer Prev. 2013;14:1077–1082. doi: 10.7314/APJCP.2013.14.2.1077. [DOI] [PubMed] [Google Scholar]
- 32.Xue S, Li QW, Che JP, Guo Y, Yang FQ, Zheng JH. Decreased expression of long non-coding RNA NBAT-1 is associated with poor prognosis in patients with clear cell renal cell carcinoma. Int J Clin Exp Pathol. 2015;8:3765–3774. [PMC free article] [PubMed] [Google Scholar]