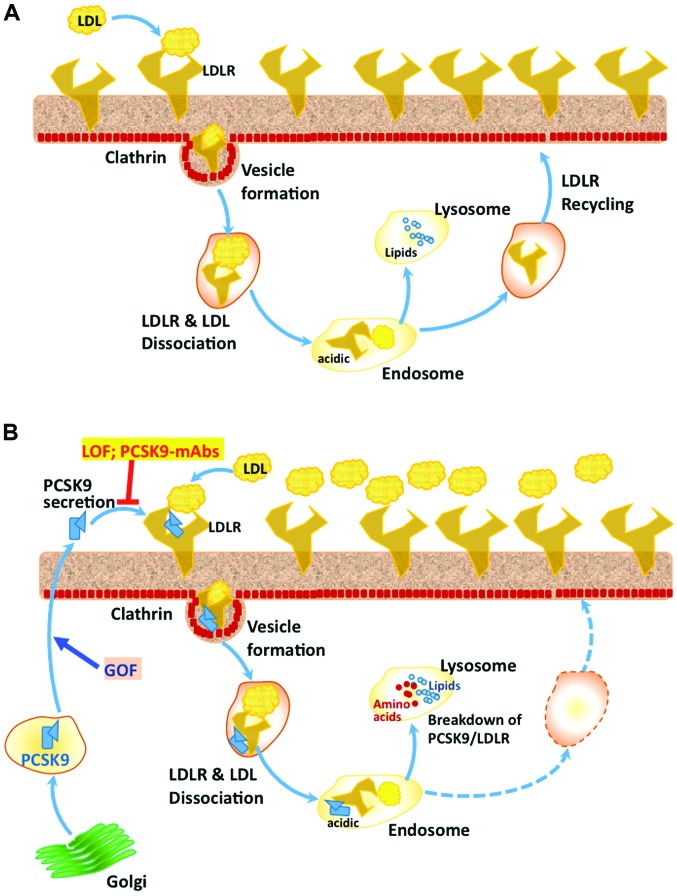
Figure 1.
PCSK9-mediated LDLR degradation pathway. (A) In the absence of bound PCSK9, following binding with LDL, LDLR on hepatocyte membrane surface is internalized through clathrin coated vesicles, which form endosomes in cytosol. The acidic environment of the endosome causes dissociation of LDLR and LDL particle, followed by the recycling of LDLR molecules back to the cell surface. However, the LDL particle is taken up by the lysosomes, where complex lipids are broken down to individual components and released into cytosol for further processing and use. (B) In the presence of PCSK9, which associates with LDLR and LDL through different domains, the complex of LDLR-PCSK9-LDL does not dissociate in the acidic environment of endosomes and is degraded into amino acids and individual lipid components, which are released into cytosol. Thus in the presence of PCSK9, there is no recycling of LDLR. Gain of function (GOF) mutations (e.g., D374Y) in PCSK9 increase its affinity for LDLR and augment its overall effect on the breakdown of LDLR. On the other hand, loss-of-function (LOF) mutations in PCSK9 (e.g., Y142X and C679X) and also treatment with therapeutic monoclonal antibodies against PCSK9 result in decreased circulating levels of PCSK9 and thus increased availability of LDLR on the cell surface and significantly lowered plasma LDL-C. PCSK9, pro-protein convertase subtilisin/kexin type 9; LDLR, low-density lipoprotein-receptor; LDL, low-density lipoprotein.