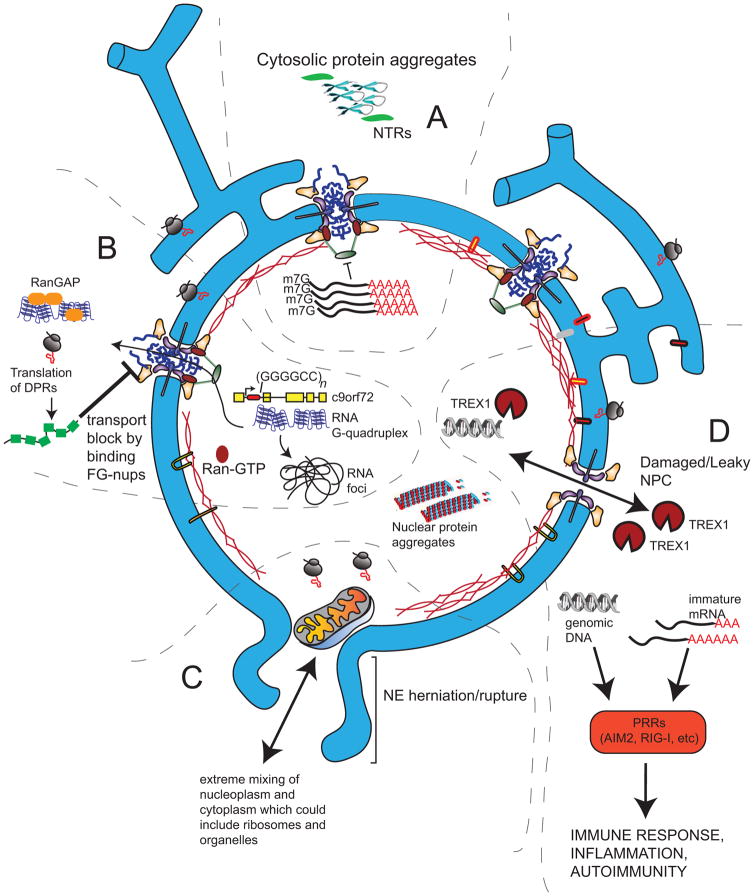
Figure 2.
Consequences of disruptions of nucleocytoplasmic compartmentalization. A. Cytosolic aggregates of β-sheet proteins can sequester NTRs and mRNA export factors, leading to the accumulation of mRNA in the nucleus. B. The transcription of the HRE of the C9orf72 intron (red) leads to the production of parallel RNA G-quadruplexes that can accumulate in RNA foci or be exported (through an unknown mechanism) into the cytosol, where they can interact with other proteins (like RanGAP) or be translated into DPRs. DPRs can direct bind to the FG-nups within the NPC to block nuclear transport. C. In more extreme circumstances a nuclear rupture event can allow even large organelles into the nucleus and will expose genomic DNA to the cytoplasm. Both cytosolic DNA and immature RNA can be recognized by pattern recognition receptors (PRRs) and illicit an immune response. D. Damage to NPCs in old neurons can lead to a breakdown of the permeability barrier and the free exchange of nuclear and cytosolic contents, leading to the accumulation of cytosolic proteins in the nucleus and nuclear-restricted nucleic acids (like immature mRNAs) in the cytosol. Cytoplasmic DNases (like TREX1) could drive DNA damage when they access the nucleoplasm.