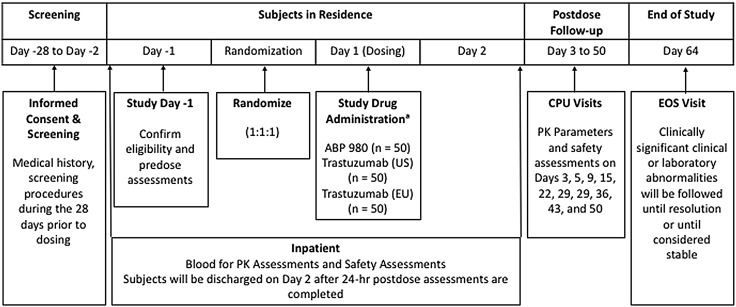
Fig. 1.
Study design. CPU clinical pharmacology unit, EOS end of study, EU European Union, FDA Food and Drug Administration, IV intravenous, PK pharmacokinetic, US United States. aPlanned IV dose: ABP 980 6 mg/kg, 440 mg vial; FDA-licensed trastuzumab 6 mg/kg, 440 mg vial; or EU-authorized trastuzumab 6 mg/kg, 150 mg vial