Fig. (4).
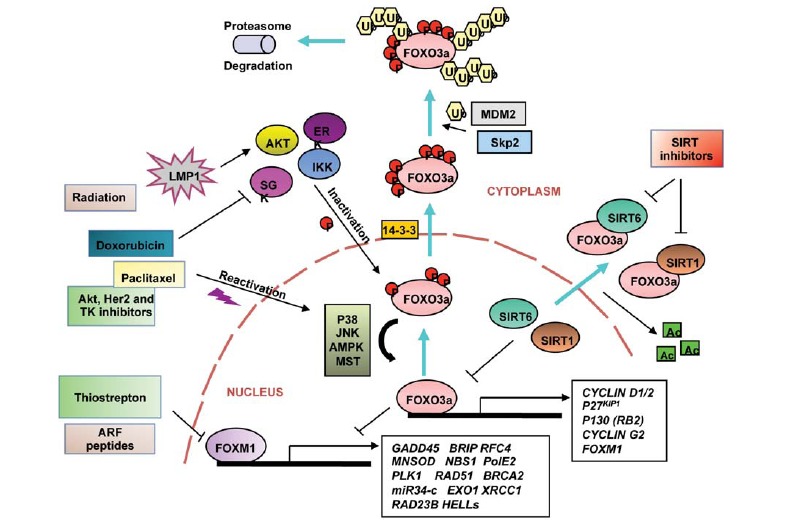
Targeting FOXO3a and FOXM1 in DNA damage response. Schematic diagramme representing upstream and downstream FOXO3a networks involved in modulation of its function and regulation of crucial transcriptional targets. FOXO3a is phosphorylated by several key oncogenic kinases such as Akt (also called PKB), IKB kinase (IKK) and serum and glucocorticoid-regulated kinase (SGK) and ERK, which facilitates recognition by Mdm2 (murine double minute 2) and Skp2 (S kinase phase protein 2) E3 ligases, leading to its nuclear exclusion, proteasome degradation and thus, inactivation of its function. The latent membrane protein 1 (LMP1), an oncoviral protein, can modulate FOXO3a expression in an Akt-dependent way to prevent DNA repair. Conversely, FOXO3a can be reactivated through phosphorylation by p38 MAPK, stress activated c-Jun-NH2-kinase (JNK), AMP-activated protein kinase (AMPK) and Ste20-like protein kinase (MST1), which are stimulated upon drug treatment or genotoxic stress such as exposure to doxorubicin, paclitaxel, UV radiation and Akt, Her2 and tyrosine kinase (TK) inhibitors. FOXO3a can also be regulated by acetylation, and SIRT 1 and SIRT6 histone deacetylase proteins play a crucial role in suppressing FOXO3a function. This phenomenon can be rescued by treatment with SIRT inhibitors, which can prevent the FOXO3a deacetylation and thus, inactivation. Cyclin D1/2 and G2, p27Kip1, p130 (RB2), GADD45, MnSOD, Polo-like kinase 1 (PLK1), Atm and the microRNA34-c are regulated by FOXO3a at the transcriptional level, modulating the processes of DNA damage response, resistance to oxidative stress, cell cycle checkpoints and senescent/quiescent state. Additionaly, FOXO3a role in regulation of the aforementioned biological processes relies, at least in part, on its ability to suppress the expression of the FOXM1 transcription factor, an important regulator of DNA repair and DNA damage response.
