Abstract
Nanomaterials can get into the blood circulation after injection or by release from implants but also by permeation of the epithelium after oral, respiratory or dermal exposure. Once in the blood, they can affect hemostasis, which is usually not intended. This review addresses effects of biological particles and engineered nanomaterials on hemostasis. The role of platelets and coagulation in normal clotting and the interaction with the immune system are described. Methods to identify effects of nanomaterials on clotting and results from in vitro and in vivo studies are summarized and the role of particle size and surface properties discussed. The literature overview showed that mainly pro-coagulative effects of nanomaterials have been described. In vitro studies suggested stronger effects of smaller than of larger NPs on coagulation and a greater importance of material than of surface charge. For instance, carbon nanotubes, polystyrene particles, and dendrimers inferred with clotting independent from their surface charge. Coating of particles with polyethylene glycol was able to prevent interaction with clotting by some particles, while it had no effect on others and the more recently developed bio-inspired surfaces might help to design coatings for more biocompatible particles. The mainly pro-coagulative action of nanoparticles could present a particular risk for individuals affected by common diseases such as diabetes, cancer, and cardiovascular diseases. Under standardized conditions, in vitro assays using human blood appear to be a suitable tool to study mechanisms of interference with hemostasis and to optimize hemocompatibility of nanomaterials.
Keywords: Nanoparticles, platelets, plasmatic coagulation, hemostasis, nanotoxicology
1. Introduction
Engineered nanoparticles (NPs) are used in a variety of applications that involve contact with the blood, such as targeted drug delivery, imaging, implants and sensors. Due to their ability to permeate epithelial barriers, particularly the air-blood barrier, NPs can reach the systemic circulation also by other routes of exposure. This contact may result in damage of erythrocytes (hemolysis), immunologic effects (uptake of NPs by monocytes, activation of complement, immunotoxicity), and in impairment of hemostasis. Although several NPs, for instance TiO2 tubes or polystyrene platelets have been developed to influence hemostasis [1, 2], for most exposures an influence on hemostasis is unwanted.
Dys-regulation of normal clotting may result in life-threatening situations, such as disseminated intravascular coagulation (DIC), also termed consumption coagulopathy and defibrination syndrome. In DIC blood clots clog the vessels and shut off blood supply of organs [3]. This results in ischemic cell damage and hemolysis. Ensuing consumption of platelets and coagulation factors causes diffuse bleeding, which worsens organ damage. DIC is induced by pro-coagulants in the blood in sepsis, severe tissue injury, obstetric complications, malignancies, liver failure, respiratory distress syndrome, and vascular disorders and is accompanied by a high mortality of 40-80%.
Also NPs may influence clotting. Epidemiological data suggest that air pollutants, for instance particulate matter (PM) are responsible for decreased as well as increased coagulation [4] but animal exposures to particles of ≤2.5 µm size (PM2.5) so far did not show significant adverse effects on blood coagulation [5].
Endothelial factors, plasmatic coagulation and platelet function regulate clotting and NPs may act on all three aspects. This review will focus on non-intended (toxic) effects of NPs on plasmatic coagulation, platelet
function, and thrombosis in vivo. Small size and cationic surface charge play an important role in cytotoxicity (for review see for instance [6]), the role of these parameters in coagulation has been studied less well. Therefore, literature data were analyzed with respect to the influence of size, surface charge, material, application route and coating on coagulation. The interaction of coagulation with the immune system was highlighted because most NPs influence immune function [7].
2. Clotting of blood (hemostasis)
Hemostasis, coagulation and clotting of blood, describe processes that prevent blood loss and bleeding. This process is regulated in the endothelial lining of the blood vessels and cellular (platelets) and soluble (plasma proteins) components from the blood.
Injury of blood vessels starts hemostasis by release of von Willebrand Factor (vWF) from endothelial cells lining the blood vessel and binding of platelets to the injured site. By contact with the injured vessel wall platelets are activated, aggregate and secrete activators to recruit other platelets. Release of Tissue Factor (TF) by endothelium and monocytes activates one of the pathways, the extrinsic pathway, of plasmatic coagulation. Extracellular matrix underneath the damaged endothelial layer, mainly collagen, binds factor XII and initiates the other pathway, the intrinsic pathway, of plasmatic coagulation. Formation of the platelet clot is often termed primary hemostasis with fibrin formation being secondary hemostasis. The process is very fast; platelets adhere to the vessel wall within 3 sec, aggregation occurs within 10 sec and fibrin formation is initiated after 5 min. After 2-3 days platelets cause clot retraction and pull the edges of the cut vessel wall together to induce healing. Normal hemostasis further includes fibrinolysis and resolution of the clot, which occurs in parallel to healing of the vessel wall.
2.1. Platelets (Thrombocytes)
These cellular components of blood clotting originate from megakaryoblasts in the bone marrow, have a round to oval shape, do not possess a nucleus and measure 2-4 µm in diameter [8]. Human platelets have a life span of about 10 days and normal blood contains 150,000-450,000 platelets/mm3. Only 67% of all platelets circulate in blood, the rest is stored in the spleen. Platelets consist of a peripheral zone with glycoprotein receptors, a structural zone with contractive microtubules, and an organelle zone with alpha granules, dense granules and lysosomes. α-Granules are 10 times more abundant than dense granules and contain large adhesive and healing proteins, hemostatic factors vWF, factor V, fibrinogen, angiogenic factors such as vascular endothelial growth factor (VEGF), angiogenin and antiangiogenic factors like for instance angiostatin and platelet factor 4 (PF-4) and β-thromboglobulin (β-TG) [9]. These granules also contain basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), stromal cell derived factor 1 (SDF1), proteases, particularly metalloproteinases 2 and 9 (MMP-2, MMP-9), necrotic factors such as tumor necrosis factor alpha and beta (TNF-α, TNF-β) and other cytokines. Dense granules contain small molecules such as ADP, serotonin, Ca2+, and histamine. Platelet activation is an energy-dependent process and requires mitochondrial function and glycogen as energy source. Platelets are involved in surveillance of vascular integrity, formation of the hemostatic plug, formation of fibrin network and healing. Main steps of primary hemostasis are adhesion of platelets, release of mediators and aggregation of platelets. Collagen exposure or thrombin action results in secretion of ADP, serotonin, fibrinogen, lysosomal enzymes and β-TG from the platelets. Serotonin mediates aggregation and vasoconstriction, various molecules released from α-granules are important for clot formation and PDGF serves for healing. Platelet activation is induced by activation of surface receptor upon binding of fibrinogen, thromboxane A2 (TXA2), Thrombin, ADP, adrenaline, collagen, antibody complexes, vWF, podoplanin and growth arrest specific gene 6 (GAS6), while binding of prostaglandin I2 (PGI2) inhibits platelet activation. Binding to the receptors activates G-protein coupled and tyrosine kinase mediated pathways (Fig. 1A). Tyrosine (Tyr)-kinase coupled receptors act through sarcoma family kinases (SFKs) and phosphoinoside-3 kinase (PI3K) (Fig. 1B). Similar to the G-protein coupled receptors these receptors use diacylglyceride (DAG) and intracellular Ca2+ changes as downstream messengers (for more details the reader is referred to reviews on this topic e.g. [10-12]). Signaling by G-proteins presents a widely used cellular pathway and the different G-protein alpha subunit isoforms are coupled to specific downstream effectors. Platelets express Gq, G12/13, Gi/z and Gs isoforms, which, with the exception of Gs, are coupled to platelet activation. The downstream regulators are assigned to specific platelet functions; Gq acts by phosphoinositid-phospholipase C - beta (PLC-β) activation on granule secretion, integrin activation, aggregation and shape changes. Gi signaling supports activation by inhibition of cAMP synthesis. G13 signaling acts via myosin light chain phosphatase kinase (MLCK) on shape and granule secretion. Small molecules secreted from granules are mainly involved in the amplification of the response. Integrin αIIbβ3 signaling initiates changes from low affinity to activated state (inside-out-signaling). This change is associated to the binding of two proteins, talin and kindlin, to the cytoplasmic region of the receptor, thereby increasing its sensitivity. Outside-in-signaling, on the other side, leads to platelet spreading, stable adhesion of platelets, granule secretion, and clot retraction.
Fig. (1).
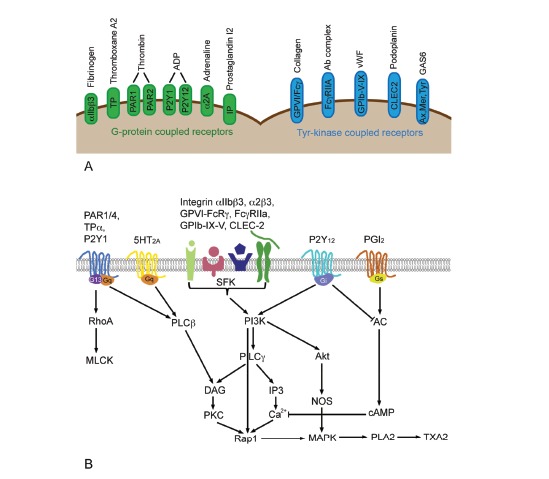
Overview of platelet surface receptors with their respective ligands (A) and the most important signaling pathways involved in platelet signaling (B). Abbreviations: AC, adenylate cyclase; cAMP, cyclic adenosylmonophosphate; CLEC-2, C-type lectin-like receptor 2; DAG, diacylglyceride; GAS6: growth arrest specific gene 6; IP, prostacyclin receptor; IP3, inositoltrisphosphate; MLCK, myosin light chain phosphatase kinase; MAPK, mitogen-activated protein kinase; PAR, protease-activated receptor; PLA2, phospholipase A2; SFK, sarcoma family kinase; TPa, thromboxane A2 receptor alpha; TXA2, thromboxane A2; vWF, von Willebrand factor.
2.2. Plasmatic Coagulation
Plasmatic coagulation is classified in intrinsic and extrinsic pathway. In normal hemostasis the primary task of the extrinsic pathway is initiating hemostasis. The intrinsic pathway acts as ‘executor’ of blood clotting but can be activated separately by various triggers, typically natural and artificial surfaces (collagen, glass, silica), prekallikrein (Fletcher factor), high molecular weight kininogen (Fitzgerald factor), and factors XII and XI [13]. Plasmatic coagulation is regulated by in total 13 factors, termed I-XIII, which are proteins with the exception of IV (calcium). The cascades serve to increase the signal in the way that each factor activates, usually by proteolytic cleavage, another factor and leads to massive amplification of the signal. Many of these factors, Prothrombin (II), Christmas factor (IX), Stuart-Power factor (X), Plasma thromboplastin antecedent (XI), and Hageman factor (XII) are serine proteases in their active forms. Fibrinogen (I) forms the fibrin network for stabilization of the initial clot consisting of platelets and is the end product of the cascade (Fig. 2). Physiological anticoagulants are antithrombin III (AT) and activated protein C (PC). Factor X links extrinsic and intrinsic pathway while thrombin connects activation of plasmatic coagulation to activation of platelets and on the other side fibrin generation to fibrinolysis. Thrombin cleaves plasminogen to active plasmin, which breaks down the links between fibrin strands and initiates degradation of the clot. Furthermore, thrombin activates PC to its active form. On the other hand, thrombin stabilizes the clot by activating thrombin-activatable fibrinolysis inhibitor (TAFI).
Fig. (2).
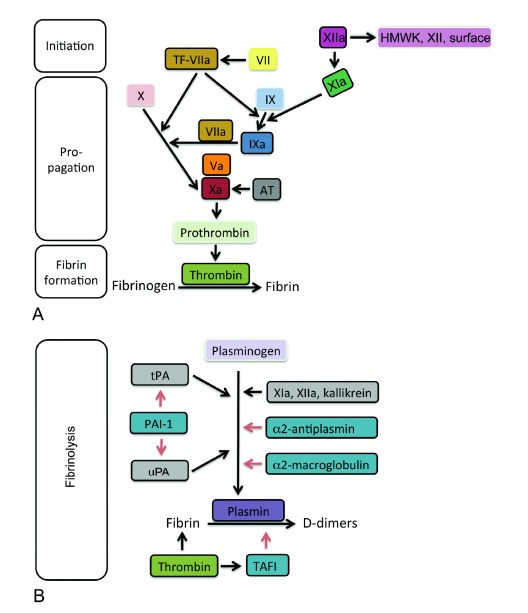
Factors of plasmatic coagulation cascade that lead to generation of the fibrin network (left) and regulators for fibrinolysis of the fibrin clot (right). Role of the specific factors to clotting (initiation, propagation and fibrin formation) is indicated. Boxes in darker shade surrounded by black lines indicate activated factors (suffix ‘a’), while squares in lighter shades of the same color and surrounded by white lines indicate the respective non-activated factors. Left: TF in combination with factor VIIa start the coagulation cascade and lead to activation of factors IX and X. Xa induces the final step prior to fibrin formation. Right: Plasminogen is activated to plasmin by tPA and uPA and by factors XIa, XIIa, and kallikrein. The activation to plasmin is inhibited indirectly by PAI-1 and directly by α2-antiplasmin and α2-macroglobulin. The degradation of fibrin to its degradation products (D-dimers) can be inhibited by TAFI. Abbreviations: TF, tissue factor; AT, antithrombin III, HMWK, high molecular weight kininogen; tPA, tissue-type plasminogen activator; PAI-1, plasminogen activator inhibitor 1; TAFI: thrombin activatable fibrinolysis inhibitor; uPA: urokinase-type plasminogen activator. Black arrows indicate activation and red arrows stand for inhibition.
Fibrinolysis is initiated by cleavage of plasminogen to plasmin and is controlled by locally released and systemic factors (Fig. 2B). Activation is stimulated by tissue-type plasminogen activator (tPA) released from endothelial cells and urokinase-type plasminogen activator (uPA). The action of tPA and uPA is inhibited by plasminogen activator inhibitor 1 (PAI-1). Kallikrein and factors XIIa and XIa start not only the intrinsic pathway of coagulation but limit also clotting via activation of plasminogen. The hepatic proteins α2-antiplasmin and α2-macroglobulin inhibit activation of plasmin.
2.3. Interaction of Coagulation and Inflammation
Activation of the extrinsic pathway of coagulation perpetuates and amplifies the inflammatory response and protects the body against spread of infectious agent [14]. This interaction is intense in sepsis and in acute arterial thrombosis (rupture of vulnerable plaques) and consists in activation of coagulation and inhibition of fibrinolysis by the inflammation process and increased secretion of cytokines and expression of adhesion molecules on endothelial cells by the coagulation process (Fig. 3).
Fig. (3).
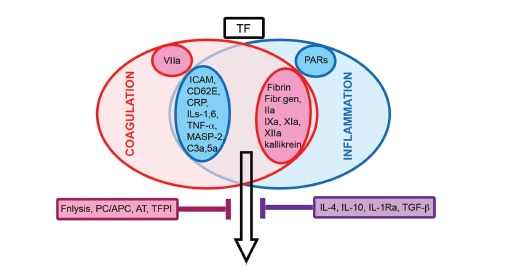
Interplay of coagulation and inflammation. Inflammation-induced endothelial cell activation with secretion of cytokines and activation of the complement system in combination with activation of the coagulation cascade act synergistically (black arrow). Increased levels of TF start both processes. Inhibition is achieved by anticoagulation (blackberry box) and anti-inflammatory (violet box) factors. Abbreviation: AT, antithrombin III; CD62E, endothelial leukocyte adhesion molecule 1; CRP, C-reactive protein; Fibr.gen, fibrinogen; Fnlysis, fibrinolysis; ICAM, intercellular adhesion molecule-1; IL, interleukin; IL-1Ra, interleukin-1 receptor antagonist; MASP-2: mannan-binding lectin serine protease 2; PARs, protease-activated receptors; PC/APC, protein C/activated protein C; TF, tissue factor; TFPI, tissue factor pathway inhibitor; TGF-ß, transforming growth factor beta.
Endothelial activation/dysfunction stimulates inflammation and coagulation due to secretion of pro-coagulants and antifibrinolytic compounds (vWF, TXA2, PAI-1). The first phase of endothelial activation includes shedding of adhesion molecules P-selectin (CD62P or GMP140), thrombin, heparin, AT, and thrombomodulin. Gene up-regulation of intercellular adhesion molecule-1 (ICAM-1), E-selectin (CD62E) and release of vWF, interleukin (IL) 8, platelet-activating factor (PAF) are part of the second phase of endothelial activation [15]. Main players in the inflammation-coagulation interaction from the coagulation side include factor VIIa belonging to the extrinsic pathway, factors IX and X of the intrinsic pathway as well as thrombin and fibrin [16]. TF, P- and E-selectin, VCAM-1, ICAM-1 stimulate neutrophilic granulocyte and platelet adhesion and promote both processes. TNF-α, IL-1, and IL-6 secreted by vascular endothelial cells and inflammatory cells amplify the hemostatic response. Activated platelets secrete adhesion molecules (fibrinogen, vWF, GPIIb/IIIa, thrombospondin, P-selectin and vitronectin), coagulation factors (fibrinogen, factors V, VII, XI, and XII), and fibrinolysis inhibitor PAI-1. Furthermore, they release microparticles (expressing TF) to stimulate coagulation. The role of MPs in coagulation will be described in the section ‘Action of biological particles on coagulation’. C-reactive protein (CRP), which can be secreted by injured blood vessels, up-regulates various endothelial adhesion molecules and induces release of IL-8 [17]. CRP inhibits platelet aggregation but induces their adhesion to endothelial cells and monocytes. CRP stimulates TF expression in monocytes, inhibits release of tPA and stimulates PAI-2 release from endothelial cells. By binding to activated platelets dissociation from the pentametic (pCRP) to monomeric (mCRP) form occurs. This conversion is important because pCRP has anti-inflammatory action, while mCRP acts pro-inflammatory and pro-thrombotic [18].
Thrombin, in addition to activation of platelets and conversion of fibrinogen to fibrin, also exerts pro-inflammatory effects by binding to protease-activated receptors (PARs) leading to up-regulation of monocyte chemotactic protein 1 (MCP-1), IL-6, IL-8, and macrophage migration inhibitory factor (MIF) by endothelial cells, platelets, and leukocytes.
Furthermore, the complement system, the most important acellular system of the unspecific immune system, intensely interacts with coagulation. Complement and coagulation display a lot of similarities: i) both cascades represent an innate defense against the invasion of external threats, such as microbia, ii) the reaction is locally restricted and very rapid, iii) most actors are serine proteases, iv) there is a tight regulation of the pathways with initiation, amplification and propagation, v) contact with foreign surfaces initiates the cascade and vi) restraint is achieved by inhibition of the actual enzyme activities and restricted binding of cascade compounds. Details on this interaction have been reviewed for instance by [19-21] and will be only cursorily addressed in this review. Main functions of the complement system are: opsonisation of pathogens, recruitment of inflammatory cells to induce elimination
of pathogens by phagocytosis or by cell lysis, and tuning of the immune response by stimulation of B- and T- cells. Activation of the complement system can occur via three pathways, the classical, the lectin and the alternative pathway. These pathways converge at the level of C3-convertase, which cleaves complement compound 3 (C3) and releases C3a and C3b [22]. C3a is a peptide mediator of inflammation and recruits phagocytes while C3b functions as opsonin and participates in the formation of C5-convertase. C5b cleaved from C5 by C5-convertase initiates the formation of the membrane attack complex, which integrates into the membrane of pathogens and induces their lysis. Interactions with coagulation occur at the level of C3. Thrombin, factors IXa, Xa and XIa, as well as kallikrein directly activate C3 [23]. Other mediators, such as kallikrein, AT, P-selectin, TAFI, TEPI and vWF are involved in coagulation and complement system [13]. Mannose-binding lectin (MBL) of the lectin pathway activates prothrombin while factor XIIa activates the classical pathway of complement activation. C3a and C5a induce exposure of P-selectin and production of TF, microparticles, cytokine and vWF by platelets. C5b-9, released from C5 upon cleavage activates platelets and stimulates cytokine secretion from endothelial cells [13]. Fibrinolysis is linked to inflammation by AT, which could be degraded by proteases released from neutrophilic granulocytes. Degradation of AT antagonizes the normal effects of AT, namely the inhibition of thrombin, and of factors Xa and IXa, the reduced activation of pro-inflammatory cells, the decreased interaction of leukocytes with endothelial cells and the induction of PGI2 in endothelial cells by the AT-thrombin complex. PC activated by platelets together with its cofactor protein S inactivates factors Va and VIIIa. It also acts anti-inflammatory by inhibition of pro-inflammatory cytokines, mainly TNF-α, IL-1β, and IL-6, secreted by monocytes, inhibition of chemotaxis and adhesion of leukocytes to endothelial cells and reduced production of pro-inflammatory cytokines and adhesion molecule. Thrombomodulin belongs to the PC system and attenuates thrombin effects, namely platelet activation, attraction of monocytes, up-regulation of leukocyte adhesion and activation of inflammatory cells [24]. The thrombin-thrombomodulin complex activates TAFI. Anti-inflammatory proteins, IL-4, IL-10, IL-1Ra, and TGF-β are additional factors in the feedback loop. C3a and C5a are inactivated by plasmin but, on the other hand, the action of plasmin on C3 and C5 proteolysis is stimulatory [23].
3. How to assess nanoparticle effects on hemostasis
Correct assessment of platelet function requires specific precautions regarding quality of the blood samples. Samples need to be obtained by clean venipuncture because ADP released upon hemolysis from erythrocytes activates platelets. Similar to patients prior to surgery [25], volunteers should not have taken non-steroidal anti-inflammatory drugs (NSAIDs) for at least 4-5 days because NSAIDs inhibit platelet function by blocking the formation of TXA2 [26]. These drugs also inhibit prostacyclin I2 synthesis by endothelial cells. Samples should be stored firmly capped to avoid CO2 loss and, in general, analysis within 3h after collection is advised. Most importantly, samples should not be cooled because this inhibits the platelet response. Contact with glass causes activation of platelets in platelet rich plasma and is another cause for false results [27].
A series of assays can be used to identify NP effects on hemostasis. Platelet activity is assessed by determination of β-TG, PF-4 and serotonin using enzyme immunoassays (EIA). The most often used enzyme immunosorbant assay (ELISA) is based on the binding of marker-specific antibodies followed by detection of the binding by a secondary horseradish peroxidase labeled antibody followed by detection of peroxidase activity [28].
Changes of platelet membrane integrity can be identified by detection of lactate dehydrogenase in the supernatant with fluorescent or colorimetric assays. Flow cytometry is the ideal technique to quantify changes in Integrin alpha-IIb (CD41), PAC1 (activated GP IIb/IIIa) and P-selectin (CD62P) expression. Flow cytometry identifies platelets according to size and granularity (side and forward scatter). The degree of expression of the respective markers is determined by fluorescence of the labeled antibodies bound to the marker. Light aggregometry is used to identify platelet aggregation and measures light transmission in stirred platelet-rich plasma or washed platelets; higher light transmission indicates aggregation.
Measurement of activated partial thromboplastin time (aPTT, activated prothrombin time) and prothrombin time (PT, partial prothrombin time, Protime, Quick’s time), Thrombin Time (Thrombin Clotting Time, TCT, TT) and Fibrinogen levels by automated analyzer are suggested to assess plasmatic coagulation [29]. aPTT and PT are assays for the activity of intrinsic and extrinsic pathway of the plasmatic coagulation, respectively. Platelet poor plasma produced from citrated blood samples is usually tested. PT is started by addition of tissue factor and calcium; aPTT with only phospholipid fraction of the tissue extract, kaolin and calcium. Prolonged clotting times in PT indicate decreased levels of factors VII, X, V, prothrombin, or fibrinogen. Deficiency of factors XII, XI, VIII, IX, X, prothrombin, and fibrinogen will lead to extended clotting times in aPTT. These clot-based assays are subjected to variations not only because they can also be performed manually instead of the automated way but also because the results depend on type of container, type of coagulant, assay compounds, collection and storage of the samples and the time between collection and analysis. aPTT and PT are also not very sensitive; a reduction of 50% of a given factor would not result in a prolonged PT and aPTT time [30]. Thrombin Time (Thrombin Clotting Time, TCT, TT) uses thrombin for induction of clotting. Clotting factors such as thrombin, tPA, uPA, factors IX, X and XII, can be determined based on the proteolytic action of Xa to cleave a chromogenic substrate. Finally, determination of D-dimer levels caused by fibrin degradation are useful for evaluation of fibrinolysis [31]. Prothrombin activation fragment (F1.2) serves as an index of in vivo thrombin generation resulting from activation of plasmatic clotting. F1.2 levels are frequently measured by ELISA. Thromboelastography (TEM® and ROTEM®) give more detailed information on clotting by measurement of time to form the fibrin clot, strength of the clot and time to lyse the fibrin clot in whole blood samples. Citrated blood is re-calcified and rotated in a cup containing a pin, which moves relative to the cup through an angle of 4°45’. Movement can either be initiated by the cup (TEM) or by the pin (ROTEM). Upon clotting fibrin strands form between cup and pin and this signal is analysed.
The above-mentioned methods were established for the clinical evaluation of patients. They are not suitable to the same extent for the evaluation of NP effects for several reasons. NPs, especially colored NPs, are known for their interference with colorimetric, fluorescent and luminescent detection by different mechanisms [32-35].
Since coagulation is assessed in protein-containing media, such as plasma, the reactive particle surface has already been coated and interference with assay compounds is less likely. Interaction by color, however, may occur and interfere with measurements of ELISA assays [36]. Analysis by flow cytometry of platelet activation is not influenced by nanoparticles because the particles are too small to be detected by this method unless the particles are fluorescent [37]. The use of appropriate assays including the right controls is essential for the evaluation of NPs on hemostasis. Some protocols have been developed by the Nanotechnology Characterization Laboratory at the National Cancer Institute-Frederick (Platelet Aggregation ITA-2 Version-1.0; Coagulation Assays, ITA-12 Version-1.0).
As mentioned before, aPTT and PT are relatively insensitive for small changes in coagulation. Also aggregometry, which has become a gold standard for the assessment of platelet aggregation might be not sensitive enough to assess the effects of NPs and a more recently developed technique based on Quartz Crystal Microbalance measurements appears to be more suitable [38].
4. Action of particles on coagulation
4.1. Effects of Biological Particles
Action of particles on coagulation is not restricted to engineered NPs. Two types of biological particles regulate coagulation and inflammation in the human body, cell-derived lipid vesicles (microparticles/exosomes) and calcium phosphate shell particles. The cell-derived lipid vesicles are classified according to their provenience and size into microparticles (MPs) and exosomes. MPs are derived from the plasma membrane in sizes of 0.1-1 µm, while exosomes originate from intracellular membranes and typically measure 40-100 nm [39]. Both types of particles are thought to be important for intercellular communication by transfer of proteins, mRNA and micro RNA (miRNA). The majority (>80%) of MPs circulating in the blood of healthy individuals are derived from platelets; endothelium-derived MPs represent 5-15%. Leukocytes are additional sources for these particles, which link coagulation, inflammation and angiogenesis. Stimulation of platelets with thrombin or shear stress induces production of MPs. Platelet-derived MPs have a life span of 10-30 min and have 50-100 fold higher pro-coagulant properties than identical surface on activated platelets. Endothelial cells release MPs after stimulation with TNF-α, Lipopolysaccharide (LPS), and oxidized low-density lipoproteins. The content of PAF, amyloid precursor protein (APP), Ca2+-dependent protease, calpain, arachidonic acid and phospholipids, and cytokine Regulated on Activation, Normal T Cell Expressed and Secreted (RANTES) is linked to their pro-inflammatory action [40]. Leukocyte-derived MPs can inhibit coagulation by expression of TAFI on their surface. Monocyte-derived MPs also express the endothelial cell protein C receptor (EPCR), thereby promoting the activation of PC by the thrombin (IIa)-thrombomodulin (TM) complex. Activated PC subsequently inactivates factors Va and VIIIa [41]. Procoagulant effects of MPs are caused by binding of coagulation factors, mainly factors VII, IX, X, and prothrombin to the negatively charged phosphatidylserine on their surface [42] but MPs can also serve as contact membranes for the activation of fibrinolysis [43]. Fibrinolysis can occur when MPs derived from endothelial cells bearing uPA/urokinase-type plasminogen activator receptor (uPAR) get into contact with plasminogen that has been bound to platelets.
The second type of biological particles is described as vesicles surrounded by a calcium phosphate shell. These particles are <500 nm, circulate in the blood, and appear to prevent coagulation [44]. Arteriosclerotic plaques, kidney and prostate stones, ovarian cancer, and altered mitral valves are potential sources for these particles. Agonist-induced platelet activation, induced by thrombin receptor activator peptide or by convulxin (activator of the collagen receptor) is inhibited by these particles. Since diseases with higher circulating levels of these particles are accompanied by hyper-coagulate state generation of these particles might be a reaction to guarantee normal hemostasis.
Engineered NPs are usually not wanted to affect hemostasis and testing for cytotoxicity, hemocompatibility and interference with the immune system are part of the biocompatibility testing. In vivo studies allow the evaluation of all aspects of hemostasis, effects on endothelium, platelet and plasmatic coagulation, as well as potential action on the immune system, while mechanistic studies are only possible to a limited degree. Importantly, the influence of LPS as common contaminant of NP preparations cannot be assessed. LPS can initiate inflammatory responses and can also influence the surface charge of NPs and biasing interpretations of effects caused by particles with different surface charges [45].
4.2. In Vivo Effects Of Engineered NPs on Coagulation
The overview of Table 1 shows that promotion of clotting by NPs has been more frequently reported than inhibition of clotting. Effects caused by parenteral application (ia, iv, ip) were not stronger than those obtained by non-parenteral application (in, it, oral). This effect is surprising because particles entering the blood directly can act more potently than particles that have to cross an epithelial barrier prior to getting in contact with blood.
Table 1.
Action of nanoparticles in vivo. Sizes of nanoparticles have been determined in different conditions (as produced and in the media in which they were applied).
| Material | Size (nm) | Effect on Platelets | Effects Plasmatic Coagulation | Effects on Thrombus Formation | Reference |
|---|---|---|---|---|---|
| Carbon, metal and metal oxide NPs | |||||
| Silver (Ag) | |||||
| ia, mouse | 15 | no effect on platelet activation | no effect on induced thrombosis | [46] | |
| it, rat | 10-100 | increased venous thrombosis | [47] | ||
| Carboxylated nanodiamonds | |||||
| iv, mouse | 4-10 | pulmonary thromboembolism | [48] | ||
| CdSe quantum dots | |||||
|
CdSe, carboxylated>amine, iv, mouse |
40 | platelet aggregation (+ agonist) | pulmonary thromboembolism | [49] | |
| Carbon nanotubes, single-walled (SWCNTs) | |||||
| ia, mouse | < 2 x 1000-5000 | platelet activation and aggregation | increase of induced thrombosis | [50] | |
| iv, rat | not given | increase of induced thrombosis | [51] | ||
| Carbon nanotubes, multi-walled (MWCNTs) | |||||
|
Amidated and carboxylated, iv, mouse |
26-31 x 490-580 | no changes in platelet counts | [52] | ||
| Iron oxide (Fe2O3) | |||||
| it, rat | 22>280 | prolonged aPTT, PT | [53] | ||
| Polystyrene (PS) | |||||
|
Carboxylated, ia, mouse |
60 | increase of induced artery occlusion time | [50] | ||
|
aminated ia, mouse |
60 | decrease of induced artery occlusion time | [50] | ||
|
aminated, carboxylated, neutral, it, hamster |
60 | no effect on induced thrombosis | [54] | ||
|
aminated, ia, hamster |
400 | no effect on induced thrombosis | [54] | ||
| Silica (SiO2), amorphous | |||||
| iv, rat | 30 | activation extrinsic pathway | [55] | ||
| ip, mouse | 50>500 | platelet aggregation | no effect on aPTT and PT | increased thrombosis | [56] |
| in, mouse | 30, 70, 100, 300, 1000 | activation intrinsic pathway | [57] | ||
| iv, mouse | 70 | interaction with factor XII | DIC | [58] | |
| iv, mouse | 100, 300, 1000 | no DIC | [58] | ||
| ia, mouse | <200 | no effect on activation | no effect on induced thrombosis | [46] | |
|
aminated, neutral oral, mouse |
200>50 | DIC | [59] | ||
| Titanium dioxide (TiO2) | |||||
|
rutile, ia, mouse |
10 x 40 | no effect on activation and aggregation | no effect on mesenterial artery occlusion time | [50] | |
|
Rutile nanorods it, rat |
4-6 | platelet aggregation | [60] | ||
|
anatase, not rutile ia, mouse |
<100 | platelet aggregation | Increase od induced thrombosis | [46] | |
| Zinc oxide (ZnO) | |||||
| ia, mouse | <110 | no platelet activation | no effect on induced thrombosis | [46] | |
| Biodegradable Nanoparticles | |||||
| Dendrimers | |||||
|
amine G7 PAMAM iv, mouse |
8.1 | fibrinogen aggregates | DIC | [61] | |
|
Amine G6.5 PAMAM oral, mouse |
8.5 | DIC | [59] | ||
|
carboxylated, hydroxylated oral, mouse |
8.5 | minimal DIC | [59] | ||
| Liposomes | |||||
|
Anionic iv, guinea pig |
not given | induction of clotting | [62] | ||
|
Cationic, neutral iv, guinea pig |
not given | no effects on coagulation | [62] | ||
|
Cationic iv, rat |
not given | thrombocytopenia | [63] | ||
Size-dependency has rarely been studied in a systematic way. One data set described effects of differently sized SiO2 particles with most prominent effects for 50-70 nm SiO2 particles. However, another study suggested that the effect of 200 nm SiO2 particles was stronger than that caused by 50 nm particles. Size-dependent effects have to be interpreted with caution because characterization was not performed in the same medium.
Consistently, dendrimers of cationic charge caused DIC while anionic particles did not. Also cationic PS particles caused more pronounced effects than anionic particles. This is in contradiction with liposomes and CdSe particles where anionic particles induced more clotting. Negatively charged surfaces are more expected to initiate thrombotic events because in physiological coagulation contact with anionic physiological surfaces (extracellular matrix) initiates clotting.
A strong contribution of inflammation for the induction of coagulation has been suggested in studies on silica particles. Intratracheal instillation of 2 µm crystalline SiO2 particles and intraperitoneal injection of 50 nm and 500 nm amorphous silica particles were evaluated for inflammation and coagulation [56, 64]. Upon both routes of application, similar trends were reported on platelet activation and plasmatic coagulation and depletion of monocytes, macrophages and neutrophilic granulocytes reduced not only inflammation but also thrombosis. Problems in comparisons of different in vivo studies include low standardization of tail bleeding and thrombosis models and strain differences in the sensitivity to the thrombosis induction. Furthermore, different doses and different ways of blood collection were used in the studies.
Taken together in vivo studies do not allow a clear statement on the role of size and surface charge on clotting.
Several studies compared in vitro effects in human blood samples to action of NPs in rodents [47, 48, 50, 51]. Only in few studies in vitro effects were more pronounced than in vivo effects [52]. Differences between in vitro samples using human blood and rodent studies would not be surprising because of differences between murine and human coagulation. Mouse blood contains much lower levels of immunologically detectable circulating PAI-1 [65], 3 times greater platelet numbers and smaller (half the size) platelets than human blood [66]. While the ultrastructure of murine platelets is similar to human platelets in most respects, the response of murine platelet to agonists differs from that of humans. Murine platelets show no response to ristocetin and need higher levels of ADP for the induction of aggregation. These differences are reflected in the evaluation of NPs. Comparison of platelet aggregation induced by the same 50 nm SiO2 particles showed that 5 µg/ml were efficient in causing a significant increase in murine platelets, while only much higher concentrations (100 µg/ml) significantly increased aggregation of human platelets [37, 67]. By using Rotation Thromboelastometry it appeared that human and ovine blood sample were more prone to coagulation than samples from pig, rats, or rabbits [68].
4.3. Effects of Engineered NPs In Vitro
In vitro assays can discriminate between particle effects on platelets and on plasmatic coagulation. NPs may activate platelets directly or interfere with agonist-induced activation. On the other hand, they can act via their surface and initiate activation of plasmatic coagulation or they can bind coagulation factors (Fig. 4).
Fig. (4).
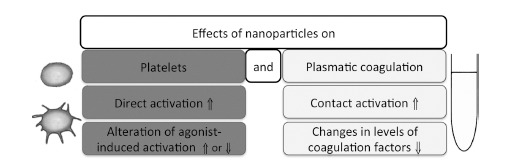
Potential effects of NPs on hemostasis. Abbreviation: ⇑: increase; ⇓: decrease
Effects of different particles are summarized in Table 2.
Table 2.
Effects of nanoparticles on clotting in vitro. Sizes of nanoparticles have been determined in different conditions (as produced and in the media in which they were applied). Data were obtained from exposure with human blood samples. Platelet activation obtained by dynamic and not in static exposure is marked by asterisk (*).
| Material | Size (nm) | Effect on Platelets | Effects Plasmatic Coagulation | Reference | |
|---|---|---|---|---|---|
| Carbon, metal and metal oxide nanoparticles | |||||
| Silver (Ag) | |||||
| 10-15 | inhibition of platelet aggregation | [69] | |||
| 16 | no effect on platelet aggregation (+/- agonist) | thrombin generation decreased, aPTT prolonged | [70] | ||
| 20 | inhibition of platelet aggregation | [71] | |||
| 24 | inhibition of intrinsic pathway | [72] | |||
| 30 | platelet activation, * | XIIa-like activity, activation of clotting | [73] | ||
| 10-100 | platelet activation and aggregation, * | [47] | |||
| 90-240 | platelet activation | induction of clot formation | [74] | ||
| Boron phosphate (BPO4), plain not folate | 180-280 | Platelet aggregation by plain, * | [75] | ||
| Gold (Au) | |||||
| solid | 9 | platelet activation | [76] | ||
| solid | 5-30 | inhibition of platelet aggregation (+/- agonist) | [77] | ||
| solid | 18>68 | platelet activation, * | [78] | ||
| solid | 18 | no effect on activation and aggregation, * | [38] | ||
| solid, cationic and anionic | 30 | no effect on platelet aggregation | [79] | ||
| solid | 50 | no effect on platelet aggregation (+/- agonist) | [80] | ||
| solid | 30, 50 | binding to fibrinogen; no effect on coagulation | [81] | ||
| solid | >60 | inhibition of platelet aggregation (+/- agonist) | [77] | ||
| solid | <60 | platelet aggregation (+ agonist) | [78] | ||
| shells | 2-3 | inhibition of platelet aggregation | [82] | ||
| shells | 15 | platelet aggregation | [82] | ||
| shells | 150 | platelet aggregation | [82] | ||
| hollow spheres | 63 | no effect on platelet aggregation (+/- agonist) | [83] | ||
| Nanodiamonds, carboxylated | 4-10 | platelet aggregation | [48] | ||
| 5;100 | no effect on PT and aPTT | [84] | |||
| CdTe, thioglycylic acid-, cystamine-capped | 2.6-4.8 | Platelet activation and aggregation, * | [85] | ||
| CdSe, cysteine-capped | 15 | platelet aggregation | [86] | ||
| C60 | |||||
| composite | 10 | no effect on platelet activation (+ agonist), * | [87] | ||
| Polyhydroxylated C60 | 7 | platelet aggregation (+ agonist) | [88] | ||
| Single-walled carbon nanotubes (SWCNTs) | |||||
| plain | not given | platelet aggregation | [51] | ||
| carboxylated> carboxylated+PEG | < 2 | platelet aggregation | accelerated clot formation | [89] | |
| Carboxylated> carboxylated+PEG | < 2 | platelet aggregation | [90] | ||
| Multi-walled carbon nanotubes (MWCNTs) | |||||
| Plain | 60-100 x 1000-2000 | platelet aggregation, release of microparticles, * | [91] | ||
| carboxylated | not given | platelet aggregation | [51] | ||
| Carboxylated, long>short | 10 x 223, 10 x 926 | platelet activation | accelerated fibrin formation, decreased clot formation, and reduced clot hardness | [92] | |
| amidated, long>short | 10 x 223, 10 x 926 | platelet activation | accelerated fibrin formation, decreased clot formation, and reduced clot hardness | [92] | |
| Plain>carboxylated | 15 x 1000-2000 | platelet aggregation | [93] | ||
| carboxylated | 26-31 x 490-580 | platelet activation | intrinsic pathway activation | [52] | |
| Amidated | 26-31 x 490-580 | platelet activation | extrinsic pathway activation | [52] | |
| Iron (Fe) | |||||
| Iron carbide, carboxylated, IgG, ProtA coated | 30 | no effect on platelet activation, * | activation of clotting: IgG and ProtA>carboxylated | [94] | |
| iron carbide, carbon coated | 33 | activation of clotting | [95] | ||
| Ferucarbotran (Resovist) | 30-90 | slight effect on activation, no effect on aggregation, * | [96] | ||
| Fe2O3; Fe3O4 | 55-65; 20-30 | no effect on platelet activation | no effect on clot formation | [74] | |
| Fe2O3 coated with poly-vinyl pyridine | 80 | intrinsic pathway inhibition | [97] | ||
| Fe3O4@nSiO2, heparin coated | 100 | no effect on PT and aPTT | [98] | ||
| Polystyrene (PS) | |||||
| carboxylated | 26 | no effect on intrinsic pathway | [99] | ||
| Carboxylated | 24, 220 | intrinsic pathway activation | [100] | ||
| aminated | 24, 220 | decreased thrombin generation by FXII, IX depletion | [100] | ||
| Neutral | 20, 200 | no effect on platelet aggregation | [93] | ||
| carboxylated, amidated | 20, 200 | platelet activation and aggregation, * | [101] | ||
| Aminated, not carboxylated | 60 | platelet activation and aggregation | [50] | ||
| neutral | 60 | no effect on activation and aggregation, * | [38] | ||
| Amidated | 60-80 | platelet aggregation | [102] | ||
| carboxylated | 60-80 | platelet activation and aggregation | [102] | ||
| Carboxylated, aminated, neutral | 50, 100 | platelet aggregation | [103] | ||
| carboxylated | 220 | intrinsic pathway activation | [99] | ||
| Anionic > cationic latex | 300 | platelet aggregation | [104] | ||
| neutral, latex | 200-400 | platelet aggregation | [105] | ||
| Silica (SiO2) | |||||
| amorphous | 15 | no effect on PT and aPTT, clotting induced | [106] | ||
| Amorphous | 10, 50, 150, 500 | platelet activation, 10>50 nm | [37] | ||
| amorphous | 30 | no effect on activation, * | no effect on clotting time | [94] | |
| Amorphous | 50 | platelet adhesion and aggregation, * | [107] | ||
| amorphous | 10, 50 | platelet activation, 10 not 50 nm, * | [38] | ||
| Amorphous, cationic and anionic | 70, 232 | induction of clotting | [108] | ||
| bare and PEGylated ORMOSIL | 35, 45 | factor XII activation, activation intrinsic pathway | [109] | ||
| Mesoporous, pluronic polymer capped | 107, 149 | no effect on PT and aPTT | [110] | ||
| Titanium dioxide (TiO2) | |||||
| Rutile | 10 x 40 | no effect on activation and aggregation | [50] | ||
| rutile | 20-160 | no effect on activation | no effect on clot formation | [74] | |
| zinc oxide (ZnO), cationic and anionic | 70, 232 | induction of clotting | [108] | ||
| Biodegradable Nanoparticles | |||||
| cetyl alcohol/polysorbate, +/- PEG, anionic | 119, 133 | inhibition of platelet aggregation | [111] | ||
| Dendrimers | |||||
| Amine G1-G4 PAMAM dendrimers | 2.2-4.5 | prolonged clotting time and decreased initial clotting velocity | [112] | ||
| carboxylated, hydroxylated, amine G3-G6 PAMAM dendrimers | 3.1-7.5 | platelet activation and aggregation, large amine, * | [113] | ||
| Amine G7 PAMAM dendrimers | 8.1 | platelet activation | platelet-dependent thrombin generation inhibited | [114] | |
| Liposomes | |||||
| Cationic | not given | inhibition of platelet aggregation (+ agonist), no effect without | [115] | ||
| anionic, neutral | not given | no effect on platelet aggregation (-/+ agonist) | [115] | ||
| Cationic | 180-200 | platelet activation | [116] | ||
| Poly(lactic-co-glycolic acid), PLGA | |||||
| PLGA-macrogol and chitosan | 100-500 | no effect on platelet aggregation (+/- agonist) | [117] | ||
| Anionic PLGA and PLGA-chitosan | 580-640 | no effect on activation and aggregation, weak inhibition of aggregation (+ agonist) | [118] | ||
| PLGA-alendronate | 200 | no effect on platelet aggregation and activation | [119] | ||
| poly(ɛ-caprolactone) lipid-core nanocapsules, chitosan coated | 476 | no effect on platelet aggregation | no effect on PT and aPTT | [120] | |
| Chitosan | |||||
| cationic | 430-580 | no effect on activation and aggregation, weak inhibition of aggregation (+ agonist), * | [118] | ||
| Chitosan, plain, PEG | 140; 105 | platelet activation of bare not of PEG-coated particles | no effect on PT and aPTT | [121] | |
Effects on platelet function have been studied more often than plasmatic coagulation. This might be due to the fact that plasmatic coagulation is usually determined by automated analysis, which is available mainly at hospitals. These organizations, on the other hand, have problems in testing experimental samples. When NPs activated plasmatic coagulation mainly the intrinsic pathway was affected, while only amidated carbon nanotubes (CNTs) activated the extrinsic pathway [52]. CNTs showed activation on platelets and plasmatic coagulation, while poly(lactic-co-glycolic acid) (PLGA) and Titanium dioxide (TiO2)- based NPs had almost no effects on platelets and plasmatic coagulation. The majority of the studies reported platelet activation and stimulation of intrinsic coagulation pathway by polystyrene (PS) particles up to 400 nm. Similar to the in vivo studies particles generally affected platelets and plasmatic coagulation in a similar way. Exceptions were iron carbide particles that affected only plasmatic coagulation and platelet activation and cationic G7 PAMAM dendrimers that activated platelets but inhibited platelet-dependent thrombin generation [94, 115].
In vitro studies suggested that small particles might act differently from larger particles. Smaller silver (Ag) NPs inhibited platelet activation and plasmatic clotting, while larger ones activated both processes. A potential explication for the controversial effect could be the dif-
ferent release of Ag+ from these particles. It has been hypothesized that the induction of platelet aggregation by silver NPs in catheter coating is caused by collision of the platelets with silver ions [122]. In general, faster release is expected from the smaller NPs due to greater surface area but coating of the particles and electrolytes in the solution can influence this release [123]. Smaller 10 nm silica (SiO2) particles showed stronger activation of platelets than 50 nm particles. Studies of solid gold (Au) particles appear to suggest that <20 nm particles induce platelet activation while larger (>30 nm) particles cause no effect but not all studies support this hypothesis. Effects of size were also noted for CNTs; long MWCNTs activated platelets stronger than shorter tubes [92].
Conflicting results were obtained when surface charge-dependent effects were compared. Most studies on CNTs reported activation of platelets and coagulation for carboxylated and amidated particles. However, one study showed that cationic CNTs induced platelet activation more strongly and that these particles activated the extrinsic pathway of coagulation while the anionic ones activated the intrinsic pathway [52]. Cationic and anionic SiO2 particles were evaluated for plasmatic coagulation, where particles of both charges induced clotting. Different results were obtained for cationic and anionic PS particles and different mechanisms were identified; anionic particles showed stronger platelet activation and activated the intrinsic pathway of plasmatic coagulation in some studies, whereas activation by cationic, not by anionic PS particles was reported in other studies [50]. Finally, some authors did not find any dependence on surface charge and reported activation of platelets for neutral, cationic and anionic PS particles [103]. Dendrimers showed platelet activation and inhibition of plasmatic coagulation. These opposite effects may contribute to DIC observed in vivo, where an over-activation of clotting results in depletion of coagulation factors. Studies on liposomes reported activation as well as inhibition of platelet function. Reasons for these disparate results comprise differences in size as well as in lipid composition. PGLA and chitosan particles in different composition excelled by lack of interference with platelet function and plasmatic coagulation.
The influence of surface charge is difficult to interpret because effective surface charge is rarely positive in physiological solution due to binding of proteins with negative charge. The interpretation of charge-dependent effects is further complicated by the fact that changes in surface charge can be accompanied by changes in hydrophobicity. Miyamoto et al. studied more and less hydrophobic latex polystyrene particles and found that the hydrophilic particles induced platelet aggregation to a lower extent than the hydrophobic ones [104]. Hydrophobic particles can interact more closely with the plasma membrane of cells [124] and this might result in platelet activation. It is also expected that coagulation factors bind to a higher extent to hydrophobic than to hydrophilic particles. These effects stimulate the immune system more strongly than hydrophilic particles. Furthermore, higher protein binding by hydrophobic particles may also cause a higher uptake by cells of the reticulo-endothelial system and influence the immune reaction this way. Hydrophilicity/-phobicity is rarely indicated in the studies. One reason for that might be the scarce availability of techniques to determine this surface property. Binding of hydrophobic dyes, for instance Bengal Rose, is only suitable for more hydrophobic particles and was not able to discriminate between PS particles of different surface charge [125]. In addition to surface charge, surface roughness, crystallinity, porosity, and chemical composition have a great influence on hemostasis. A variety of techniques for instance atomic force microscopy (AFM), transmission and scanning electron microscopy (TEM, SEM), small angle x-ray scattering (SAXS), X-ray diffraction (XRD), Brunauer, Emmett, Teller method (BET), etc. are available to determine these parameters [126]. However, these data are not routinely assessed and comparison between the data obtained by different techniques is difficult.
Differences between in vitro studies could also be caused by use of different platelet preparations; washed platelets were used in few studies (for instance [51]) while the majority studied effects in platelet-rich plasma. The interaction with plasma proteins has been shown to decrease platelet activation [85]. Further differences included the use of dynamic exposure (stirring, shaker) versus static exposure. Table 2 suggests, however, that platelet activation was not linked to the exposure condition. Particles tested in flow and in static condition showed the same effect on platelet activation [85].
Several mechanisms have been postulated to explain the increased clotting in NP exposed cells. Denaturation of factor XII by larger SiO2 particles but not by smaller particles has been postulated [127]. On the other hand, consistent with the experimental data, a higher degree of contact activation of factor XII has been described for 30-50 nm particles due to the greater access to the surface [57]. In one study SiO2 particles induced the intrinsic pathway of coagulation, while in another study, silica NPs of slightly greater size activated the extrinsic pathway [58]. MWCNTs, depending on the surface charge, showed activation by different routes; activation of factor IX, activation related to factor XII, and activation of the plasmatic coagulation by platelet activation [52]. Activation of integrin αIIbβ3 signaling represented an important mechanism for the activation of platelets by PS, SWCNTs, MWCNTs, silica, C60 fullerenes and CdTe quantum dots as well as for the inhibition by Ag NPs [51, 69, 85, 103, 128]. Surface charge did not have a prominent effect on this activation [85, 103].
5. How to prevent adverse effects on hemostasis
Biological effects usually are contributed to the binding of macromolecules, mainly proteins, from physiological media [129]. Interference with the immune system has been linked to the degree of protein binding to particles and prevention of this binding can decrease unwanted biological effects [130]. Coating of NPs with bulky molecules, commonly PEG, poly(vinylalcohol) (PVA), ethylenediaminetetraacetate (EDTA), polyethylene oxide (PEO), dextran sulfate, decreases protein binding to NPs as well as phagocytosis [121]. These coatings have also been used to decrease interference with platelets and coagulation factors. PEGylated SWCNTs caused less platelet aggregation than uncoated carboxylated particles, which is consistent with the idea that prevention of protein binding decreases interference with hemostasis [89, 90]. Coating with PEG, however, was not successful for all materials. No effect of PEGylation was observed for Au particles and SWCNTs [52, 90, 131]. The relative inefficacy of PEGylation for SWCNTs was also seen in studies on complement activation and suggests that obtaining a sufficient coating of these particles may be difficult [132, 133]. Similarly, PEGylation of ORMOSIL did not change the pro-coagulant effect of these particles [109, 111]. It appears that PEGylation of particles is not suitable for all particles and particle-specific coatings need to be developed. Coating with starch reduced platelet aggregation to iron oxide NPs [134]. A stealth formulation consisting of PEG and dipalmitoylphosphatidylcholine (DPPC) reduced platelet activation and inhibited agonist (ADP) induced activation of polymeric particles [135]. For the development of biocompatible NPs, recent strategies on bio-inspired particles could be extremely useful. Bio-inspired particles were able to fulfill specific roles in the organism. Hydrogels mimicking red blood cells regarding size, shape and surface charge showed less non-intended interaction with blood cells and removal by the reticulo-endothelial system [136]. Similarly, NPs with platelet shape and different surface coatings could interact with biological substrates in a controlled manner and polydopamine coated micro- and nanoparticles, in contrast to uncoated PS particles, did not influence platelet aggregation and activation [2, 137]. Furthermore, information obtained from biocompatibility of implant surfaces could be used for the design of NPs [138].
Conclusion
Activation of platelets and induction of intrinsic pathway of plasmatic coagulation are the common mechanisms for increased clotting induced by NPs. This mainly pro-coagulate action of NPs could be particularly dangerous for individuals suffering from hyper-coagulate states linked to common diseases, such as diabetes mellitus, arteriosclerosis, cancer and obstructive pulmonary disease. Thromboembolism in cancer patients occurs 6 times more frequently than in healthy individuals [139]. Hyper-coagulation is due to transformation of macrophages into tumor-type macrophages, which activate angiogenesis and promote tumor cell proliferation by secretion of pro-coagulant cytokines. These macrophages also impair fibrinolysis by increased levels of uPA and PAI-1 and decrease of tPA concentrations. Arteriosclerotic lesions are thrombogenic due to release of TF by macrophages and smooth muscle cells. In addition, fibrinolysis is impaired due to increased levels of uPA and PAI-1 [140]. Insulin resistance in diabetes patients increases TF and factor VII production [141]. Increased blood levels of prothrombin, fibrinogen and PAI-1 in combination with glycosylation and oxidation of clotting factors facilitate clot formation. Pro-coagulant levels in diabetes patients are higher than in healthy controls [142]. Inflammatory processes in the respiratory tract are major promoters of coagulation in asthma patients [143]. Impaired function of the PC pathway in combination with increased activation of platelets can cause pulmonary thrombosis. In parallel, increased production of PAI-1 causes decreased fibrinolysis of the formed thrombi. Other respiratory diseases, for instance chronic obstructive pulmonary disease and cystic fibrosis, display the same prothrombotic changes and make patients more vulnerable to the effects of NPs. This assumption is supported by the higher incidence of thrombotic events in regions with increased concentrations of PM in the air [144], where individuals with pre-existing alterations of coagulation were more affected.
The correlation of hemostatic effects to particle parameters based on in vitro studies indicated some importance of size on platelet activation. In vivo studies, on the other hand, did not show a clear correlation of size or surface-charge to coagulation. The described effects were in part material-specific. It might be concluded that hydrophobicity and surface topology could be more relevant for coagulation than size and surface charge.
ACKNOWLEDGEMENTS
Support of the studies by the Austrian Science Fund grants N 214-NAN and P 22576-B18 and by the European integrated project NMP4-CT-2006-026723 is gratefully acknowledged.
LIST OF ABBREVIATIONS
- AC
adenylate cyclase
- AFM
atomic force microscope
- APC
activated protein C
- APP
amyloid precursor protein
- aPTT
activated partial thromboplastin time
- AT
antithrombin III
- BET
Brunauer, Emmett, Teller method
- bFGF
basic fibroblast growth factor
- C3
complement compound 3
- cAMP
cyclic adenosylmonophosphate
- CdSe
cadmium selenide
- CLEC-2
C-type lectin-like receptor 2
- CRP
C-reactive protein
- DAG
diacylglyceride
- DIC
disseminated intravascular coagulation
- DPPC
dipalmitoylphosphatidylcholine
- EDTA
ethylenediaminetetraacetate
- EIA
enzyme Immunoassay
- ELISA
enzyme linked immunosorbent Assay
- EPCR
endothelial cell protein C receptor
- F1.2
prothrombin activation fragment
- GAS6
growth arrest specific gene 6
- HMWK
high molecular weight kininogen
- ia
intra-arterial
- ICAM-1
intercellular adhesion molecule-1
- IgG
immunoglobulin G
- IL
interleukin
- IL-1Ra
interleukin-1 receptor antagonist
- in
intranasal
- ip
intraperitoneal
- IP
prostacyclin receptor
- IP3
inositoltrisphosphate
- it
intratracheal
- iv
intravenous
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemotactic protein 1
- MIF
macrophage migration inhibitory factor
- miRNA
micro RNA
- MLCK
myosin light chain phosphatase kinase
- MMP
metalloproteinase
- MPs
microparticles
- MWCNTs
multi-walled carbon nanotubes
- NP
nanoparticle
- NSAIDs
non-steroidal anti-inflammatory drugs
- PAF
platelet-activating factor
- PAI-1
plasminogen activator inhibitor 1
- PAMAM
Polyamidoamine
- PAR
protease-activated receptor
- PC
protein C
- PEG
polyethylene glycol
- PEO
polyethylene oxide
- PF-4
platelet factor 4
- PGI2
prostaglandin I2
- PI3K
phosphoinoside-3 kinase
- PLA2
phospholipase A2
- PLC-β
phosphoinositide-phospholipase - beta
- PLGA
poly(lactic-co-glycolic acid)
- PM
particulate matter
- ProtA
protein A
- PS
polystyrene
- PT
partial prothrombin time
- RANTES
regulated on activation, normal T cell expressed and secreted
- SAXS
small angle x-ray scattering
- SDF-1
stromal cell derived factor 1
- SEM
scanning electron microscope
- SFK
sarcoma family kinase
- SiO2
silica
- SWCNTs
single-walled carbon nanotubes
- TAFI
thrombin-activatable fibrinolysis inhibitor
- TEM
transmission electron microscope
- TF
tissue factor
- TFPI
tissue factor pathway inhibitor
- TGF-β
transforming growth factor beta
- TiO2
titanium dioxide
- TM
thrombin (IIa)-thrombomodulin
- TNF-α
Tumor necrosis factor alpha
- TP
thromboxane receptor
- tPA
tissue-type plasminogen activator
- TT
thrombin Time
- TXA2
thromboxane A2
- uPA
urokinase-type plasminogen activator
- uPAR
urokinase-type plasminogen activator receptor
- VCAM-1
vascular cell adhesion molecule 1
- vWF
Willebrand Factor
- XRD
X-ray diffraction
- β-TG
β- thromboglobulin
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
References
- 1.Roy S.C., Paulose M., Grimes C.A. The effect of TiO2 nanotubes in the enhancement of blood clotting for the control of hemorrhage. Biomaterials. 2007;28(31):4667–4672. doi: 10.1016/j.biomaterials.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 2.Anselmo A.C., Modery-Pawlowski C.L., Menegatti S., Kumar S., Vogus D.R., Tian L.L., Chen M., Squires T.M., Sen Gupta A., Mitragotri S. Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano. 2014;8(11):11243–11253. doi: 10.1021/nn503732m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schafer A. Hemorrhagic Disorders: DIC, Liver Failure, and Vitamin K Deficiency. In: Goldman L., Schafer A., editors. Goldman's Cecil Medicine. Philadelphia: Elsevier Saunders; 2011. [Google Scholar]
- 4.Lippi G., Favaloro E.J., Franchini M., Guidi G.C. Air pollution and coagulation testing: a new source of biological variability? Thromb. Res. 2008;123(1):50–54. doi: 10.1016/j.thromres.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Nadziejko C., Fang K., Chen L.C., Cohen B., Karpatkin M., Nadas A. Effect of concentrated ambient particulate matter on blood coagulation parameters in rats. Res. Rep. Health Eff. Inst. 2002;(111):7–29. [PubMed] [Google Scholar]
- 6.Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomedicine. 2012;7:5577–5591. doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fröhlich E. Value of phagocyte function screening for immunotoxicity of nanoparticles in vivo. Int. J. Nanomedicine. 2015;10:3761–3778. doi: 10.2147/IJN.S83068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews R.K., Berndt M.C. Platelet physiology and thrombosis. Thromb. Res. 2004;114(5-6):447–453. doi: 10.1016/j.thromres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Stalker T.J., Traxler E.A., Wu J., Wannemacher K.M., Cermignano S.L., Voronov R., Diamond S.L., Brass L.F. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121(10):1875–1885. doi: 10.1182/blood-2012-09-457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senis Y.A., Mazharian A., Mori J. Src family kinases: at the forefront of platelet activation. Blood. 2014;124(13):2013–2024. doi: 10.1182/blood-2014-01-453134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z., Delaney M.K., O'Brien K.A., Du X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010;30(12):2341–2349. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brass L.F., Tomaiuolo M., Stalker T.J. Harnessing the platelet signaling network to produce an optimal hemostatic response. Hematol. Oncol. Clin. North Am. 2013;27(3):381–409. doi: 10.1016/j.hoc.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conway E.M. Reincarnation of ancient links between coagulation and complement. J. Thromb. Haemost. 2015;13(Suppl. 1):S121–S132. doi: 10.1111/jth.12950. [DOI] [PubMed] [Google Scholar]
- 14.Levi M., van der Poll T., Buller H.R. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109(22):2698–2704. doi: 10.1161/01.CIR.0000131660.51520.9A. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J., Defelice A.F., Hanig J.P., Colatsky T. Biomarkers of endothelial cell activation serve as potential surrogate markers for drug-induced vascular injury. Toxicol. Pathol. 2010;38(6):856–871. doi: 10.1177/0192623310378866. [DOI] [PubMed] [Google Scholar]
- 16.Zoller B., Li X., Sundquist J., Sundquist K. Autoimmune diseases and venous thromboembolism: a review of the literature. Am. J. Cardiovasc. Dis. 2012;2(3):171–183. [PMC free article] [PubMed] [Google Scholar]
- 17.Fay W.P. Linking inflammation and thrombosis: Role of C-reactive protein. World J. Cardiol. 2010;2(11):365–369. doi: 10.4330/wjc.v2.i11.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenhardt S.U., Habersberger J., Murphy A., Chen Y.C., Woollard K.J., Bassler N., Qian H., von Zur Muhlen C., Hagemeyer C.E., Ahrens I., Chin-Dusting J., Bobik A., Peter K. Dissociation of pentameric to monomeric C-reactive protein on activated platelets localizes inflammation to atherosclerotic plaques. Circ. Res. 2009;105(2):128–137. doi: 10.1161/CIRCRESAHA.108.190611. [DOI] [PubMed] [Google Scholar]
- 19.Oikonomopoulou K., Ricklin D., Ward P.A., Lambris J.D. Interactions between coagulation and complement--their role in inflammation. Semin. Immunopathol. 2012;34(1):151–165. doi: 10.1007/s00281-011-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markiewski M.M., Nilsson B., Ekdahl K.N., Mollnes T.E., Lambris J.D. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28(4):184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Amara U., Rittirsch D., Flierl M., Bruckner U., Klos A., Gebhard F., Lambris J.D., Huber-Lang M. Interaction between the coagulation and complement system. Adv. Exp. Med. Biol. 2008;632:71–79. doi: 10.1007/978-0-387-78952-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy K., Travers P., Walport M. Janeway's Immunobiology. 7th ed. New York: Garland Science; 2008. [Google Scholar]
- 23.Amara U., Flierl M.A., Rittirsch D., Klos A., Chen H., Acker B., Bruckner U.B., Nilsson B., Gebhard F., Lambris J.D., Huber-Lang M. Molecular intercommunication between the complement and coagulation systems. J. Immunol. 2010;185(9):5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margetic S. Inflammation and hemostasis. Biochem. Med. 2012;22(1):49–62. [PMC free article] [PubMed] [Google Scholar]
- 25.Younan M., Atkinson T., Fudin J. A practical approach to discontinuing NSAID therapy prior to a procedure. Pract. Pain Manag. 2013;13(10):45–51. [Google Scholar]
- 26.Schafer A.I. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J. Clin. Pharmacol. 1995;35(3):209–219. doi: 10.1002/j.1552-4604.1995.tb04050.x. [DOI] [PubMed] [Google Scholar]
- 27.Harrison P., Mackie I., Mumford A., Briggs C., Liesner R., Winter M., Machin S. British Committee for Standards in, H. Guidelines for the laboratory investigation of heritable disorders of platelet function. Br. J. Haematol. 2011;155(1):30–44. doi: 10.1111/j.1365-2141.2011.08793.x. [DOI] [PubMed] [Google Scholar]
- 28.Nix B., Wild D. Data Processing. In: Gosling J., editor. Immunoassays, A Practical Approach. Oxford: Oxford University Press; 2000. pp. 239–261. [Google Scholar]
- 29.Evani S., Ramasubramanian A. 2011. Hemocompatibility of Nanoparticles. [Google Scholar]
- 30.Monroe D., III, Hoffman M., Roberts H. Molecular Biology and Biochemistry of the Coagulation Factors and Pathways of Hemostasis. In: Kaushansky K., Lichtman M., Seligsohn U., Beutler E., Kipps T., Prchal J., editors. Williams Hematology. 8th ed. New York: McGraw-Hill Companies; 2010. [Google Scholar]
- 31.Riley R., Tidwell A., Williams D., Bode A., Carr M. Laboratory evaluation of hemostasis. http://www.pathology.vcu.edu/clinical/coag/Lab Hemostasis.pdf. [March 2015].
- 32.Fröhlich E., Meindl C., Pieber T. Important issues in the cytotoxicity screening of nano-sized materials. EURO-NanoTox Lett. 2010;1:1–6. [Google Scholar]
- 33.Kong B., Seog J.H., Graham L.M., Lee S.B. Experimental considerations on the cytotoxicity of nanoparticles. Nanomedicine: Nanotech. Biol. Med. 2011;6(5):929–941. doi: 10.2217/nnm.11.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroll A., Pillukat M.H., Hahn D., Schnekenburger J. Current in vitro methods in nanoparticle risk assessment: limitations and challenges. Eur. J. Pharm. Biopharm. 2009;72(2):370–377. doi: 10.1016/j.ejpb.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Monteiro-Riviere N.A., Inman A.O., Zhang L.W. Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line. Toxicol. Appl. Pharmacol. 2009;234(2):222–235. doi: 10.1016/j.taap.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 36.Kroll A., Pillukat M.H., Hahn D., Schnekenburger J. Interference of engineered nanoparticles with in vitro toxicity assays. Arch. Toxicol. 2012;86(7):1123–1136. doi: 10.1007/s00204-012-0837-z. [DOI] [PubMed] [Google Scholar]
- 37.Corbalan J.J., Medina C., Jacoby A., Malinski T., Radomski M.W. Amorphous silica nanoparticles aggregate human platelets: potential implications for vascular homeostasis. Int. J. Nanomedicine. 2012;7:631–639. doi: 10.2147/IJN.S28293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santos-Martinez M.J., Inkielewicz-Stepniak I., Medina C., Rahme K., D'Arcy D.M., Fox D., Holmes J.D., Zhang H., Radomski M.W. The use of quartz crystal microbalance with dissipation (QCM-D) for studying nanoparticle-induced platelet aggregation. Int. J. Nanomedicine. 2012;7:243–255. doi: 10.2147/IJN.S26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burnier L., Fontana P., Kwak B.R., Angelillo-Scherrer A. Cell-derived microparticles in haemostasis and vascular medicine. Thromb. Haemost. 2009;101(3):439–451. [PubMed] [Google Scholar]
- 40.Owens A.P., III, Mackman N. Microparticles in hemostasis and thrombosis. Circ. Res. 2011;108(10):1284–1297. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Angelillo-Scherrer A. Leukocyte-derived microparticles in vascular homeostasis. Circ. Res. 2012;110(2):356–369. doi: 10.1161/CIRCRESAHA.110.233403. [DOI] [PubMed] [Google Scholar]
- 42.Markiewicz M., Richard E., Marks N., Ludwicka-Bradley A. Impact of endothelial microparticles on coagulation, inflammation, and angiogenesis in age-related vascular diseases. 2013. [DOI] [PMC free article] [PubMed]
- 43.Plawinski L., Angles-Cano E. Membrane microvesicles: a circulating source for fibrinolysis, new antithrombotic messengers. Haematologica. 2013;98(7):e75–e76. doi: 10.3324/haematol.2013.088948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller V.M., Hunter L.W., Chu K., Kaul V., Squillace P.D., Lieske J.C., Jayachandran M. Biologic nanoparticles and platelet reactivity. Nanomedicine (Lond.) 2009;4(7):725–733. doi: 10.2217/nnm.09.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ilinskaya A.N., Dobrovolskaia M.A. Nanoparticles and the blood coagulation system. Part II: safety concerns. Nanomedicine (Lond.) 2013;8(6):969–981. doi: 10.2217/nnm.13.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haberl N., Hirn S., Holzer M., Zuchtriegel G., Rehberg M., Krombach F. Effects of acute systemic administration of TiO, ZnO, SiO, and Ag nanoparticles on hemodynamics, hemostasis and leukocyte recruitment. Nanotoxicology. 2015:1–9. doi: 10.3109/17435390.2014.992815. [DOI] [PubMed] [Google Scholar]
- 47.Jun E.A., Lim K.M., Kim K., Bae O.N., Noh J.Y., Chung K.H., Chung J.H. Silver nanoparticles enhance thrombus formation through increased platelet aggregation and procoagulant activity. Nanotoxicology. 2011;5(2):157–167. doi: 10.3109/17435390.2010.506250. [DOI] [PubMed] [Google Scholar]
- 48.Kumari S., Singh M.K., Singh S.K., Gracio J.J., Dash D. Nanodiamonds activate blood platelets and induce thromboembolism. Nanomedicine (Lond.) 2014;9(3):427–440. doi: 10.2217/nnm.13.23. [DOI] [PubMed] [Google Scholar]
- 49.Geys J., Nemmar A., Verbeken E., Smolders E., Ratoi M., Hoylaerts M.F., Nemery B., Hoet P.H. Acute toxicity and prothrombotic effects of quantum dots: impact of surface charge. Environ. Health Perspect. 2008;116(12):1607–1613. doi: 10.1289/ehp.11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bihari P., Holzer M., Praetner M., Fent J., Lerchenberger M., Reichel C.A., Rehberg M., Lakatos S., Krombach F. Single-walled carbon nanotubes activate platelets and accelerate thrombus formation in the microcirculation. Toxicology. 2010;269(2-3):148–154. doi: 10.1016/j.tox.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 51.Radomski A., Jurasz P., Alonso-Escolano D., Drews M., Morandi M., Malinski T., Radomski M.W. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br. J. Pharmacol. 2005;146(6):882–893. doi: 10.1038/sj.bjp.0706386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burke A.R., Singh R.N., Carroll D.L., Owen J.D., Kock N.D., D'Agostino R., Jr, Torti F.M., Torti S.V. Determinants of the thrombogenic potential of multiwalled carbon nanotubes. Biomaterials. 2011;32(26):5970–5978. doi: 10.1016/j.biomaterials.2011.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu M.T., Feng W.Y., Wang B., Wang T.C., Gu Y.Q., Wang M., Wang Y., Ouyang H., Zhao Y.L., Chai Z.F. Comparative study of pulmonary responses to nano- and submicron-sized ferric oxide in rats. Toxicology. 2008;247(2-3):102–111. doi: 10.1016/j.tox.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 54.Nemmar A., Hoylaerts M.F., Hoet P.H., Vermylen J., Nemery B. Size effect of intratracheally instilled particles on pulmonary inflammation and vascular thrombosis. Toxicol. Appl. Pharmacol. 2003;186(1):38–45. doi: 10.1016/s0041-008x(02)00024-8. [DOI] [PubMed] [Google Scholar]
- 55.Liu X., Sun J. Time-course effects of intravenously administrated silica nanoparticles on blood coagulation and endothelial function in rats. J. Nanosci. Nanotechnol. 2013;13(1):222–228. doi: 10.1166/jnn.2013.6910. [DOI] [PubMed] [Google Scholar]
- 56.Nemmar A., Albarwani S., Beegam S., Yuvaraju P., Yasin J., Attoub S., Ali B.H. Amorphous silica nanoparticles impair vascular homeostasis and induce systemic inflammation. Int. J. Nanomedicine. 2014;9:2779–2789. doi: 10.2147/IJN.S52818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshida T., Yoshioka Y., Tochigi S., Hirai T., Uji M., Ichihashi K., Nagano K., Abe Y., Kamada H., Tsunoda S., Nabeshi H., Higashisaka K., Yoshikawa T., Tsutsumi Y. Intranasal exposure to amorphous nanosilica particles could activate intrinsic coagulation cascade and platelets in mice. Part. Fibre Toxicol. 2013;10:41. doi: 10.1186/1743-8977-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nabeshi H., Yoshikawa T., Matsuyama K., Nakazato Y., Arimori A., Isobe M., Tochigi S., Kondoh S., Hirai T., Akase T., Yamashita T., Yamashita K., Yoshida T., Nagano K., Abe Y., Yoshioka Y., Kamada H., Imazawa T., Itoh N., Kondoh M., Yagi K., Mayumi T., Tsunoda S., Tsutsumi Y. Amorphous nanosilicas induce consumptive coagulopathy after systemic exposure. Nanotechnology. 2012;23(4):045101. doi: 10.1088/0957-4484/23/4/045101. [DOI] [PubMed] [Google Scholar]
- 59.Greish K., Thiagarajan G., Herd H., Price R., Bauer H., Hubbard D., Burckle A., Sadekar S., Yu T., Anwar A., Ray A., Ghandehari H. Size and surface charge significantly influence the toxicity of silica and dendritic nanoparticles. Nanotoxicology. 2012;6(7):713–723. doi: 10.3109/17435390.2011.604442. [DOI] [PubMed] [Google Scholar]
- 60.Nemmar A., Melghit K., Ali B.H. The acute proinflammatory and prothrombotic effects of pulmonary exposure to rutile TiO2 nanorods in rats. Exp. Biol. Med. 2008;233(5):610–619. doi: 10.3181/0706-RM-165. [DOI] [PubMed] [Google Scholar]
- 61.Jones C.F., Campbell R.A., Brooks A.E., Assemi S., Tadjiki S., Thiagarajan G., Mulcock C., Weyrich A.S., Brooks B.D., Ghandehari H., Grainger D.W. Cationic PAMAM dendrimers aggressively initiate blood clot formation. ACS Nano. 2012;6(11):9900–9910. doi: 10.1021/nn303472r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zbinden G., Wunderli-Allenspach H., Grimm L. Assessment of thrombogenic potential of liposomes. Toxicology. 1989;54(3):273–280. doi: 10.1016/0300-483x(89)90063-2. [DOI] [PubMed] [Google Scholar]
- 63.Reinish L., Bally M., Loughrey H., Cullis P. Interactions of liposomes and platelets. Thromb. Haemost. 2008;60(3):518–523. [PubMed] [Google Scholar]
- 64.Nemmar A., Nemery B., Hoet P.H., Van Rooijen N., Hoylaerts M.F. Silica particles enhance peripheral thrombosis: key role of lung macrophage-neutrophil cross-talk. Am. J. Respir. Crit. Care Med. 2005;171(8):872–879. doi: 10.1164/rccm.200409-1202OC. [DOI] [PubMed] [Google Scholar]
- 65.Casanave E., Tentoni J. Comparative Fibrinolysis. In: Kolev K., editor. Fibrinolysis and Thrombolysis. USA: InTech; 2014. [Google Scholar]
- 66.Ware J. Dysfunctional platelet membrane receptors: from humans to mice. Thromb. Haemost. 2004;92(3):478–485. doi: 10.1267/THRO04090478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nemmar A., Yuvaraju P., Beegam S., Yasin J., Dhaheri R.A., Fahim M.A., Ali B.H. In vitro platelet aggregation and oxidative stress caused by amorphous silica nanoparticles. Int. J. Physiol. Pathophysiol. Pharmacol. 2015;7(1):27–33. [PMC free article] [PubMed] [Google Scholar]
- 68.Siller-Matula J.M., Plasenzotti R., Spiel A., Quehenberger P., Jilma B. Interspecies differences in coagulation profile. Thromb. Haemost. 2008;100(3):397–404. [PubMed] [Google Scholar]
- 69.Shrivastava S., Bera T., Singh S.K., Singh G., Rama-chandrarao P., Dash D. Characterization of antiplatelet properties of silver nanoparticles. ACS Nano. 2009;3(6):1357–1364. doi: 10.1021/nn900277t. [DOI] [PubMed] [Google Scholar]
- 70.Laloy J., Minet V., Alpan L., Mullier F., Beken S., Toussaint O., Lucas S., Dogné J. Impact of silver nanoparticles on haemolysis, platelet function and coagulation. Nanobiomedicine. 2014;1(4) doi: 10.5772/59346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ragaseema V.M., Unnikrishnan S., Kalliyana Krishnan V., Krishnan L.K. The antithrombotic and antimicrobial properties of PEG-protected silver nanoparticle coated surfaces. Biomaterials. 2012;33(11):3083–3092. doi: 10.1016/j.biomaterials.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 72.Martinez-Gutierrez F., Thi E.P., Silverman J.M., de Oliveira C.C., Svensson S.L., Vanden Hoek A., Sanchez E.M., Reiner N.E., Gaynor E.C., Pryzdial E.L., Conway E.M., Orrantia E., Ruiz F., Av-Gay Y., Bach H. Antibacterial activity, inflammatory response, coagulation and cytotoxicity effects of silver nanoparticles. Nanomedicine (Lond.) 2012;8(3):328–336. doi: 10.1016/j.nano.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 73.Krajewski S., Prucek R., Panacek A., Avci-Adali M., Nolte A., Straub A., Zboril R., Wendel H.P., Kvitek L. Hemocompatibility evaluation of different silver nanoparticle concentrations employing a modified Chandler-loop in vitro assay on human blood. Acta Biomater. 2013;9(7):7460–7468. doi: 10.1016/j.actbio.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 74.Guildford A.L., Poletti T., Osbourne L.H., Di Cerbo A., Gatti A.M., Santin M. Nanoparticles of a different source induce different patterns of activation in key biochemical and cellular components of the host response. J. R. Soc. Interface. 2009;6(41):1213–1221. doi: 10.1098/rsif.2009.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Achilli C., Grandi S., Ciana A., Guidetti G.F., Malara A., Abbonante V., Cansolino L., Tomasi C., Balduini A., Fagnoni M., Merli D., Mustarelli P., Canobbio I., Balduini C., Minetti G. Biocompatibility of functionalized boron phosphate (BPO4) nanoparticles for boron neutron capture therapy (BNCT) application. Nanomedicine (Lond.) 2014;10(3):589–597. doi: 10.1016/j.nano.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 76.Wiwanitkit V., Sereemaspun A., Rojanathanes R. Gold nanoparticles and a microscopic view of platelets: a preliminary observation. Cardiovasc. J. Afr. 2009;20(2):141–142. [PubMed] [Google Scholar]
- 77.Aseychev A.V., Azizova O.A., Beckman E.M., Dudnik L.B., Sergienko V.I. Effect of gold nanoparticles coated with plasma components on ADP-induced platelet aggregation. Bull. Exp. Biol. Med. 2013;155(5):685–688. doi: 10.1007/s10517-013-2226-x. [DOI] [PubMed] [Google Scholar]
- 78.Deb S., Patra H.K., Lahiri P., Dasgupta A.K., Chakrabarti K., Chaudhuri U. Multistability in platelets and their response to gold nanoparticles. Nanomedicine (Lond.) 2011;7(4):376–384. doi: 10.1016/j.nano.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 79.Love S.A., Thompson J.W., Haynes C.L. Development of screening assays for nanoparticle toxicity assessment in human blood: preliminary studies with charged Au nanoparticles. Nanomedicine (Lond.) 2012;7(9):1355–1364. doi: 10.2217/nnm.12.17. [DOI] [PubMed] [Google Scholar]
- 80.Solomon A., Smyth E., Mitha N., Pitchford S., Vydyanath A., Luther P.K., Thorley A.J., Tetley T.D., Emerson M. Induction of platelet aggregation after a direct physical interaction with diesel exhaust particles. J. Thromb. Haemost. 2013;11(2):325–334. doi: 10.1111/jth.12087. [DOI] [PubMed] [Google Scholar]
- 81.Dobrovolskaia M.A., Patri A.K., Zheng J., Clogston J.D., Ayub N., Aggarwal P., Neun B.W., Hall J.B., McNeil S.E. Interaction of colloidal gold nanoparticles with human blood: effects on particle size and analysis of plasma protein binding profiles. Nanomedicine (Lond.) 2009;5(2):106–117. doi: 10.1016/j.nano.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Akchurin G., Akchurin G., Ivanov A., Kirichuk V., Terentyuk G., Khlebtsov B., Khlebtsov N. In: Vo-Dinh T., Lakowicz J., editors. Proc. SPIE Influence of Gold Nanoparticles on Platelets Functional Activity In Vitro. In: Plasmonics in Biology and Medicine ; San Jose. 2008. p. 68690V. [Google Scholar]
- 83.You J., Zhou J., Zhou M., Liu Y., Robertson J.D., Liang D., Van Pelt C., Li C. Pharmacokinetics, clearance, and biosafety of polyethylene glycol-coated hollow gold nanospheres. Part. Fibre Toxicol. 2014;11:26. doi: 10.1186/1743-8977-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mona J., Kuo C.J., Perevedentseva E., Priezzhev A., Cheng C. Adsorption of human blood plasma on nanodiamond and its influence on activated partial thromboplastin time. Diamond Related Materials. 2013;39:73–77. [Google Scholar]
- 85.Samuel S.P., Santos-Martinez M.J., Medina C., Jain N., Radomski M.W., Prina-Mello A., Volkov Y. CdTe quantum dots induce activation of human platelets: implications for nanoparticle hemocompatibility. Int. J. Nanomedicine. 2015;10:2723–2734. doi: 10.2147/IJN.S78281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dunpall R., Nejo A.A., Pullabhotla V.S., Opoku A.R., Revaprasadu N., Shonhai A. An in vitro assessment of the interaction of cadmium selenide quantum dots with DNA, iron, and blood platelets. IUBMB Life. 2012;64(12):995–1002. doi: 10.1002/iub.1100. [DOI] [PubMed] [Google Scholar]
- 87.Vaschenko V.I., Samonin V.V., Popov V.A., Antonenkova E.V., Nikonova V.J., Vilyaninov V.N., Podvjaznikov M.L., Kidalov V.N., Titulova T.B., Vaschenko T.N. Effects of fullerene C60 nanocomposites on human platelet aggregation. Bull. Exp. Biol. Med. 2012;152(5):624–626. doi: 10.1007/s10517-012-1592-0. [DOI] [PubMed] [Google Scholar]
- 88.Niwa Y., Iwai N. Nanomaterials induce oxidized low-density lipoprotein cellular uptake in macrophages and platelet aggregation. Circ. J. 2007;71(3):437–444. doi: 10.1253/circj.71.437. [DOI] [PubMed] [Google Scholar]
- 89.Sokolov A., Aseychev A., Kostevich V., Gusev A., Gusev S., Vlasova I. Functionalization of single-walled carbon nanotubes regulates their effect on hemostasis. J. Phys. Conf. Ser. 2011;291(1):012054. [Google Scholar]
- 90.Vakhrusheva T.V., Gusev A.A., Gusev S.A., Vlasova I. Albumin reduces thrombogenic potential of single-walled carbon nanotubes. Toxicol. Lett. 2013;221(2):137–145. doi: 10.1016/j.toxlet.2013.05.642. [DOI] [PubMed] [Google Scholar]
- 91.De Paoli S.H., Diduch L.L., Tegegn T.Z., Orecna M., Strader M.B., Karnaukhova E., Bonevich J.E., Holada K., Simak J. The effect of protein corona composition on the interaction of carbon nanotubes with human blood platelets. Biomaterials. 2014;35(24):6182–6194. doi: 10.1016/j.biomaterials.2014.04.067. [DOI] [PubMed] [Google Scholar]
- 92.Meng J., Cheng X., Liu J., Zhang W., Li X., Kong H., Xu H. Effects of long and short carboxylated or aminated multiwalled carbon nanotubes on blood coagulation. PLoS One. 2012;7(7):e38995. doi: 10.1371/journal.pone.0038995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Semberova J., De Paoli L.S., Simakova O., Holada K., Gelderman M., Simak J. Carbon nanotubes activate blood platelets by inducing extracellular Ca2+ influx sensitive to calcium entry inhibitors. Nano Lett. 2009;9:3312–3317. doi: 10.1021/nl901603k. [DOI] [PubMed] [Google Scholar]
- 94.Bircher L., Theusinger O., Locher S., Eugster P., Roth-Z'graggen B., Schumacher C., Studt J., Stark W., Beck-Schimmer B., Herrmann I. Characterization of carbon-coated magnetic nanoparticles using clinical blood coagulation assays: effect of PEG-functionalization and comparison to silica nanoparticles. J. Mater. Chem. B Mater. Biol. Med. 2014;2:3753–3758. doi: 10.1039/c4tb00208c. [DOI] [PubMed] [Google Scholar]
- 95.Herrmann I.K., Urner M., Hasler M., Roth-Z'Graggen B., Aemisegger C., Baulig W., Athanassiou E.K., Regenass S., Stark W.J., Beck-Schimmer B. Iron core/shell nanoparticles as magnetic drug carriers: possible interactions with the vascular compartment. Nanomedicine (Lond.) 2011;6(7):1199–1213. doi: 10.2217/nnm.11.33. [DOI] [PubMed] [Google Scholar]
- 96.Aurich K., Spoerl M.C., Furll B., Sietmann R., Greinacher A., Hosten N., Weitschies W. Development of a method for magnetic labeling of platelets. Nanomedicine (Lond.) 2012;8(5):537–544. doi: 10.1016/j.nano.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 97.Ali L.M., Gutierrez M., Cornudella R., Moreno J.A., Pinol R., Gabilondo L., Millan A., Palacio F. Hemostasis disorders caused by polymer coated iron oxide nanoparticles. J. Biomed. Nanotechnol. 2013;9(7):1272–1285. doi: 10.1166/jbn.2013.1637. [DOI] [PubMed] [Google Scholar]
- 98.Zhang Y., Liu X., Lu Y., Wang J., Dong T., Liu X. Fabrication of heparinized mesoporous silica nanoparticles as multifunctional drug carriers. 2013.
- 99.Sanfins E., Augustsson C., Dahlback B., Linse S., Cedervall T. Size-dependent effects of nanoparticles on enzymes in the blood coagulation cascade. Nano Lett. 2014;14(8):4736–4744. doi: 10.1021/nl501863u. [DOI] [PubMed] [Google Scholar]
- 100.Oslakovic C., Cedervall T., Linse S., Dahlback B. Polystyrene nanoparticles affecting blood coagulation. Nanomedicine (Lond.) 2012;8(6):981–986. doi: 10.1016/j.nano.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 101.Mayer A., Vadon M., Rinner B., Novak A., Wintersteiger R., Fröhlich E. The role of nanoparticle size in hemocompatibility. Toxicology. 2009;258:139–147. doi: 10.1016/j.tox.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 102.McGuinnes C., Duffin R., Brown S.N., Mills L.N., Megson I.L., Macnee W., Johnston S., Lu S.L., Tran L., Li R., Wang X., Newby D.E., Donaldson K. Surface derivatization state of polystyrene latex nanoparticles determines both their potency and their mechanism of causing human platelet aggregation in vitro. Toxicol. Sci. 2011;119(2):359–368. doi: 10.1093/toxsci/kfq349. [DOI] [PubMed] [Google Scholar]
- 103.Smyth E., Solomon A., Vydyanath A., Luther P.K., Pitchford S., Tetley T.D., Emerson M. Induction and enhancement of platelet aggregation in vitro and in vivo by model polystyrene nanoparticles. Nanotoxicology. 2014:1–9. doi: 10.3109/17435390.2014.933902. [DOI] [PubMed] [Google Scholar]
- 104.Miyamoto M., Sasakawa S., Ozawa T., Kawaguchi H., Ohtsuka Y. Mechanisms of blood coagulation induced by latex particles and the roles of blood cells. Biomaterials. 1990;11(6):385–388. doi: 10.1016/0142-9612(90)90091-4. [DOI] [PubMed] [Google Scholar]
- 105.Movat H.Z., Weiser W.J., Glynn M.F., Mustard J.F. Platelet phagocytosis and aggregation. J. Cell Biol. 1965;27(3):531–543. doi: 10.1083/jcb.27.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Laloy J., Robert S., Marbehant C., Mullier F., Mejia J., Piret J.P., Lucas S., Chatelain B., Dogne J.M., Toussaint O., Masereel B., Rolin S. Validation of the calibrated thrombin generation test (cTGT) as the reference assay to evaluate the procoagulant activity of nanomaterials. Nanotoxicology. 2012;6(2):213–232. doi: 10.3109/17435390.2011.569096. [DOI] [PubMed] [Google Scholar]
- 107.Kim D., Finkenstaedt-Quinn S., Hurley K.R., Buchman J.T., Haynes C.L. On-chip evaluation of platelet adhesion and aggregation upon exposure to mesoporous silica nanoparticles. Analyst (Lond.) 2014;139(5):906–913. doi: 10.1039/c3an01679j. [DOI] [PubMed] [Google Scholar]
- 108.Steuer H., Krastev R., Lembert N. Metallic oxide nanoparticles stimulate blood coagulation independent of their surface charge. J. Biomed. Mater. Res. B Appl. Biomater. 2014;102(5):897–902. doi: 10.1002/jbm.b.33051. [DOI] [PubMed] [Google Scholar]
- 109.Tavano R., Segat D., Reddi E., Kos J., Rojnik M., Kocbek P., Iratni S., Scheglmann D., Colucci M., Echevarria I.M., Selvestrel F., Mancin F., Papini E. Procoagulant properties of bare and highly PEGylated vinyl-modified silica nanoparticles. Nanomedicine (Lond.) 2010;5(6):881–896. doi: 10.2217/nnm.10.65. [DOI] [PubMed] [Google Scholar]
- 110.Yildirim A., Demirel G.B., Erdem R., Senturk B., Tekinay T., Bayindir M. Pluronic polymer capped biocompatible mesoporous silica nanocarriers. Chem. Commun. (Camb.) 2013;49(84):9782–9784. doi: 10.1039/c3cc45967e. [DOI] [PubMed] [Google Scholar]
- 111.Koziara J.M., Oh J.J., Akers W.S., Ferraris S.P., Mumper R.J. Blood compatibility of cetyl alcohol/polysorbate-based nanoparticles. Pharm. Res. 2005;22(11):1821–1828. doi: 10.1007/s11095-005-7547-7. [DOI] [PubMed] [Google Scholar]
- 112.Markowicz-Piasecka M., Luczak E., Chalubinski M., Broncel M., Mikiciuk-Olasik E., Sikora J. Studies towards biocompatibility of PAMAM dendrimers--overall hemostasis potential and integrity of the human aortic endothelial barrier. Int. J. Pharm. 2014;473(1-2):158–169. doi: 10.1016/j.ijpharm.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 113.Dobrovolskaia M.A., Patri A.K., Simak J., Hall J.B., Semberova J., De Paoli Lacerda S.H., McNeil S.E. Nanoparticle size and surface charge determine effects of PAMAM dendrimers on human platelets in vitro. Mol. Pharm. 2012;9(3):382–393. doi: 10.1021/mp200463e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jones C.F., Campbell R.A., Franks Z., Gibson C.C., Thiagarajan G., Vieira-de-Abreu A., Sukavaneshvar S., Mohammad S.F., Li D.Y., Ghandehari H., Weyrich A.S., Brooks B.D., Grainger D.W. Cationic PAMAM dendrimers disrupt key platelet functions. Mol. Pharm. 2012;9(6):1599–1611. doi: 10.1021/mp2006054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Juliano R.L., Hsu M.J., Peterson D., Regen S.L., Singh A. Interactions of conventional or photopolymerized liposomes with platelets in vitro. Exp. Cell Res. 1983;146(2):422–427. doi: 10.1016/0014-4827(83)90144-1. [DOI] [PubMed] [Google Scholar]
- 116.Strieth S., Nussbaum C.F., Eichhorn M.E., Fuhrmann M., Teifel M., Michaelis U., Berghaus A., Dellian M. Tumor-selective vessel occlusions by platelets after vascular targeting chemotherapy using paclitaxel encapsulated in cationic liposomes. Int. J. Cancer. 2008;122(2):452–460. doi: 10.1002/ijc.23088. [DOI] [PubMed] [Google Scholar]
- 117.Ramtoola Z., Lyons P., Keohane K., Kerrigan S.W., Kirby B.P., Kelly J.G. Investigation of the interaction of biodegradable micro- and nanoparticulate drug delivery systems with platelets. J. Pharm. Pharmacol. 2011;63(1):26–32. doi: 10.1111/j.2042-7158.2010.01174.x. [DOI] [PubMed] [Google Scholar]
- 118.Li X., Radomski A., Corrigan O.I., Tajber L., De Sousa Menezes F., Endter S., Medina C., Radomski M.W. Platelet compatibility of PLGA, chitosan and PLGA-chitosan nanoparticles. Nanomedicine (Lond.) 2009;4(7):735–746. doi: 10.2217/nnm.09.65. [DOI] [PubMed] [Google Scholar]
- 119.Cenni E., Granchi D., Avnet S., Fotia C., Salerno M., Micieli D., Sarpietro M.G., Pignatello R., Castelli F., Baldini N. Biocompatibility of poly(D,L-lactide-co-glycolide) nanoparticles conjugated with alendronate. Biomaterials. 2008;29(10):1400–1411. doi: 10.1016/j.biomaterials.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 120.Bender E.A., Adorne M.D., Colome L.M., Abdalla D.S., Guterres S.S., Pohlmann A.R. Hemocompatibility of poly(varepsilon-caprolactone) lipid-core nanocapsules stabilized with polysorbate 80-lecithin and uncoated or coated with chitosan. Int. J. Pharm. 2012;426(1-2):271–279. doi: 10.1016/j.ijpharm.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 121.Nadesh R., Narayanan D.P, R.S., Vadakumpully S., Mony U., Koyakkutty M., Nair S.V., Menon D. Hematotoxicological analysis of surface-modified and -unmodified chitosan nanoparticles. J. Biomed. Mater. Res. A. 2013;101(10):2957–2966. doi: 10.1002/jbm.a.34591. [DOI] [PubMed] [Google Scholar]
- 122.Stevens K.N., Crespo-Biel O., van den Bosch E.E., Dias A.A., Knetsch M.L., Aldenhoff Y.B., van der Veen F.H., Maessen J.G., Stobberingh E.E., Koole L.H. The relationship between the antimicrobial effect of catheter coatings containing silver nanoparticles and the coagulation of contacting blood. Biomaterials. 2009;30(22):3682–3690. doi: 10.1016/j.biomaterials.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 123.Reidy B., Haase A., Luch A., Dawson K., Lynch I. Mechanisms of silver nanoparticle release, transformation and toxicity: a critical review of current knowledge and recommendations for future studies and applications. Materials (Basel) 2013;6:2295–2350. doi: 10.3390/ma6062295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kou L., Sun J., Zhai Y., He Z. The endocytosis and intracellular fate of nanomedicines: Implication for rational design. Asian J. Pharm. Sci. 2013;8(1):1–10. [Google Scholar]
- 125.Meindl C., Kueznik T., Bösch M., Roblegg E., Fröhlich E. Intracellular calcium levels as screening tool for nanoparticle toxicity. J. Appl. Toxicol. 2015;35(10):1150–1159. doi: 10.1002/jat.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Meyer-Plath A., Schweinsberger F. Nanomaterial Characterization and Metrology. In: Malsch I., Emond C., editors. Nanotechnology and Human Health. Boca Raton: CRC Press; 2014. [Google Scholar]
- 127.Kushida T., Saha K., Subramani C., Nandwana V., Rotello V.M. Effect of nano-scale curvature on the intrinsic blood coagulation system. Nanoscale. 2014;6(23):14484–14487. doi: 10.1039/c4nr04128c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guidetti G.F., Consonni A., Cipolla L., Mustarelli P., Balduini C., Torti M. Nanoparticles induce platelet activation in vitro through stimulation of canonical signalling pathways. Nanomedicine (Lond.) 2012;8(8):1329–1336. doi: 10.1016/j.nano.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 129.Lundqvist M., Stigler J., Elia G., Lynch I., Cedervall T., Dawson K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA. 2008;105(38):14265–14270. doi: 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dobrovolskaia M.A., McNeil S.E. Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines. J. Control. Release. 2013;172(2):456–466. doi: 10.1016/j.jconrel.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Santos-Martinez M.J., Rahme K., Corbalan J.J., Faulkner C., Holmes J.D., Tajber L., Medina C., Radomski M.W. Pegylation increases platelet biocompatibility of gold nanoparticles. J. Biomed. Nanotechnol. 2014;10(6):1004–1015. doi: 10.1166/jbn.2014.1813. [DOI] [PubMed] [Google Scholar]
- 132.Hamad I., Hunter A.C., Szebeni J., Moghimi S.M. Poly(ethylene glycol)s generate complement activation products in human serum through increased alternative pathway turnover and a MASP-2-dependent process. Mol. Immunol. 2008;46(2):225–232. doi: 10.1016/j.molimm.2008.08.276. [DOI] [PubMed] [Google Scholar]
- 133.Andersen A.J., Windschiegl B., Ilbasmis-Tamer S., Degim I.T., Hunter A.C., Andresen T.L., Moghimi S.M. Complement activation by PEG-functionalized multi-walled carbon nanotubes is independent of PEG molecular mass and surface density. Nanomedicine (Lond.) 2013;9(4):469–473. doi: 10.1016/j.nano.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 134.Deb S., Raja S.O., Dasgupta A.K., Sarkar R., Chattopadhyay A.P., Chaudhuri U., Guha P., Sardar P. Surface tunability of nanoparticles in modulating platelet functions. Blood Cells Mol. Dis. 2012;48(1):36–44. doi: 10.1016/j.bcmd.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 135.Khanbeigi R., Hashim Z., Abelha T., Pitchford S., Collins H., Green M., Dailey L. Interactions of stealth conjugated polymer nanoparticles with human whole blood. J. Mater. Chem. B Mater. Biol. Med. 2015;3:2463–2471. doi: 10.1039/c4tb01822b. [DOI] [PubMed] [Google Scholar]
- 136.Merkel T.J., Jones S.W., Herlihy K.P., Kersey F.R., Shields A.R., Napier M., Luft J.C., Wu H., Zamboni W.C., Wang A.Z., Bear J.E., DeSimone J.M. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc. Natl. Acad. Sci. USA. 2011;108(2):586–591. doi: 10.1073/pnas.1010013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ye W., Shi Q., Hou J., Gao J., Li C., Jin J., Shi H., Yin J. Fabricating bio-inspired micro/nano-particles by polydopamine coating and surface interactions with blood platelets. Appl. Surf. Sci. 2015;351:236–242. [Google Scholar]
- 138.Thevenot P., Hu W., Tang L. Surface chemistry influence implant biocompatibility. Curr. Top. Med. Chem. 2008;8(4):270–280. doi: 10.2174/156802608783790901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bagatin D., Sturm D., Bagatin T. Changes of coagulation factors in patients with carcinomas and carcinomas of the breast; perioperative influence of local anesthetics. Period. Biol. 2013;115(2):299–302. [Google Scholar]
- 140.Hoffmeister H.M., Heller W., Seipel L. Z. Kardiol. 1999;88(5):315–323. doi: 10.1007/s003920050292. [Blood coagulation and fibrinolysis in arteriosclerosis]. [DOI] [PubMed] [Google Scholar]
- 141.Alzahrani S., Ajjan R. Coagulation and fibrinolysis in diabetes. Diab. Vasc. Dis. Res. 2010;7(4):260–273. doi: 10.1177/1479164110383723. [DOI] [PubMed] [Google Scholar]
- 142.Carr M.E. Diabetes mellitus: a hypercoagulable state. J. Diabetes Complications. 2001;15(1):44–54. doi: 10.1016/s1056-8727(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 143.de Boer J.D., Majoor C.J., van 't Veer C., Bel E.H., van der Poll T. Asthma and coagulation. Blood. 2012;119(14):3236–3244. doi: 10.1182/blood-2011-11-391532. [DOI] [PubMed] [Google Scholar]
- 144.Baccarelli A., Martinelli I., Zanobetti A., Grillo P., Hou L.F., Bertazzi P.A., Mannucci P.M., Schwartz J. Exposure to particulate air pollution and risk of deep vein thrombosis. Arch. Intern. Med. 2008;168(9):920–927. doi: 10.1001/archinte.168.9.920. [DOI] [PMC free article] [PubMed] [Google Scholar]


