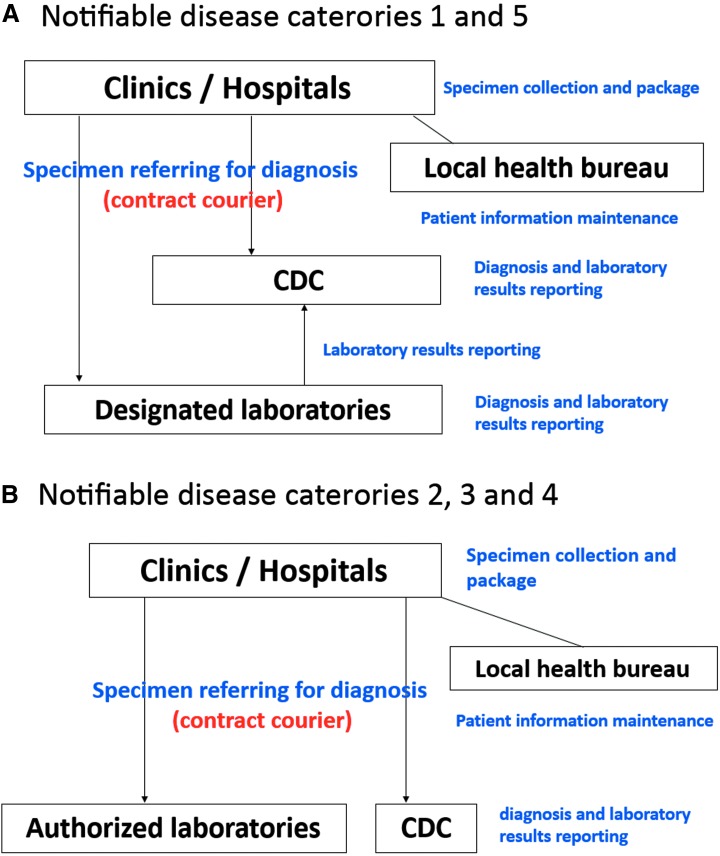
Figure 3.
Specimen referral and diagnosis networks of notifiable diseases in Taiwan. The networks for notifiable diseases belonging to (A) categories 1 and 5 as well as to (B) categories 2, 3, and 4 are illustrated. (A) Clinical specimens of patients with suspected highly infectious newly emerging (categories 1 or 5) diseases are required to be collected and packaged from local clinics and hospitals and sent to designated or TCDC laboratories for diagnosis. Positive results detected by designated laboratories are confirmed by TCDC before final report announcement. (B) Regional and district authorized laboratories serve as diagnostic agencies for categories 2, 3, and 4 diseases. However, each laboratory has the authority to announce positive results without TCDC confirmation. All the personal and epidemiologic information of each suspected or laboratory-confirmed patient is maintained by the local health bureaus through a web-based system.