Figure 2.
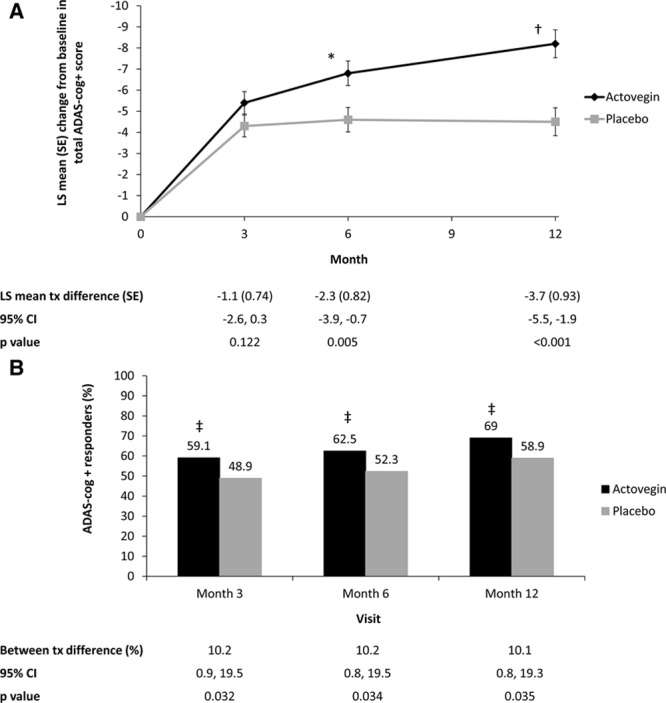
Analysis of the effect of Actovegin and placebo on Alzheimer’s Disease Assessment Scale, cognitive subscale, extended version (ADAS-cog+). A, Change in ADAS-cog+ score from baseline over the course of the study in the intent-to-treat (ITT) population. B, Analysis of ADAS-cog+ responders in the ITT population. A responder was defined as demonstrating an improvement of ≥4 points on the ADAS-cog+ scale using observed case data. *P=0.005; †P<0.001; ‡P<0.05 vs placebo. CI indicates confidence interval; LS, least squares; and tx, treatment.
