FIG 1.
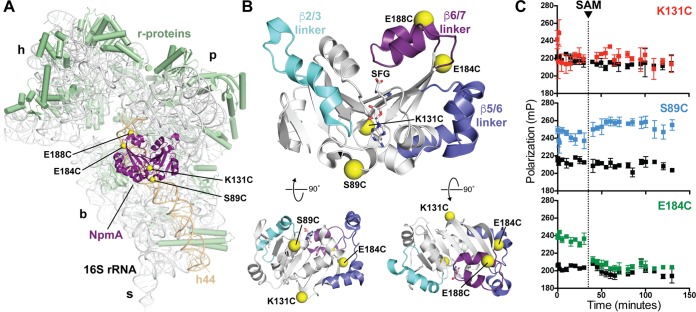
NpmA structure and development of a 30S-NpmA binding assay. (A) View of NpmA (purple) bound to the 30S subunit. Locations of unique Cys residues in NpmA incorporated for site-specific fluorescein labeling are shown as yellow spheres. Ribosomal proteins are shown in green and 16S rRNA in white, except helix 44 (h44), which is highlighted in tan. 30S features are labeled as head (h), platform (p), base (b), and stalk (s). (B) NpmA structure in three orthogonal views (top orientation is viewed from the 30S subunit, i.e., ∼180° rotation around the y axis from panel A). Sites of label incorporation are shown as described for panel A, and the NpmA β2/3 (cyan), β5/6 (slate), and β6/7 (purple) linkers are also highlighted. (C) Pilot analyses comparing FP signal for labeled NpmA variants before and after addition of SAM (noted by the dotted vertical line), either in the presence of 30S or alone (colored black for all three proteins). Only NpmA E184C* (green; bottom plot) exhibits increased FP in the presence of 30S and decreased FP after SAM addition, indicative of initial binding and subsequent dissociation of the enzyme following catalysis of methyl transfer, respectively.