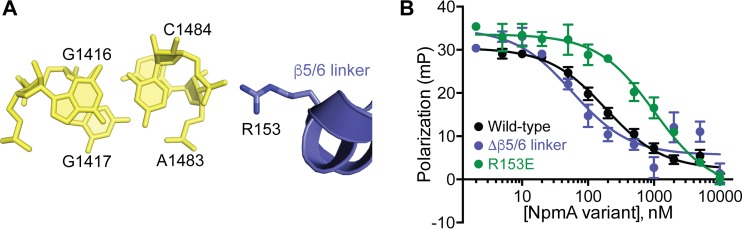
FIG 5.
R153 contributes to 30S-NpmA binding affinity. (A) View of NpmA β5/6 linker residue R153 interaction with the phosphate group bridging 16S rRNA nucleotides A1483 and C1484. (B) Competition FP binding experiments with NpmA-E184C* and unlabeled charge reversal substitution of NpmA residue 153 (R153E). The wild-type NpmA and NpmA-Δβ5/6 data, shown for comparison, are the same as those shown in Fig. 2 and 4, respectively. Error bars represent the SEM. Binding affinity (Ki) for NpmA-R153E derived from these data are shown in Table 1.