FIG 3.
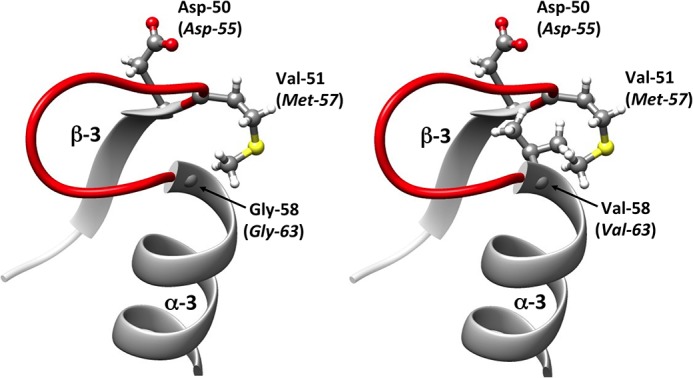
Representation of the secondary structures surrounding the aspartate residue (Asp) at position 50/55. The protein variant was modeled from the crystallographic structure of the DrrB response regulator of Thermotoga maritima (PDB entry 1P2F) by the introduction of an amino acid substitution followed by energy minimization. The amino acids belong to DrrB, whereas those italicized in parentheses are their corresponding residues in the homolog E. coli OmpR protein. The secondary structure in red refers to the loop lying between the α-3 helix and the β-3 strand. (Left) Regulator response DrrB of Thermotoga maritima with glycine residue at position 58 (corresponding to the Gly-63 residue in the native OmpR). (Right) Variant of DrrB with a Gly-58-Val substitution, corresponding to the Gly-63-Val replacement in the OmpR-G63V variant.
