EXTENDED DATA FIGURE 8. Astrocyte-derived toxic factor promoting cell death.
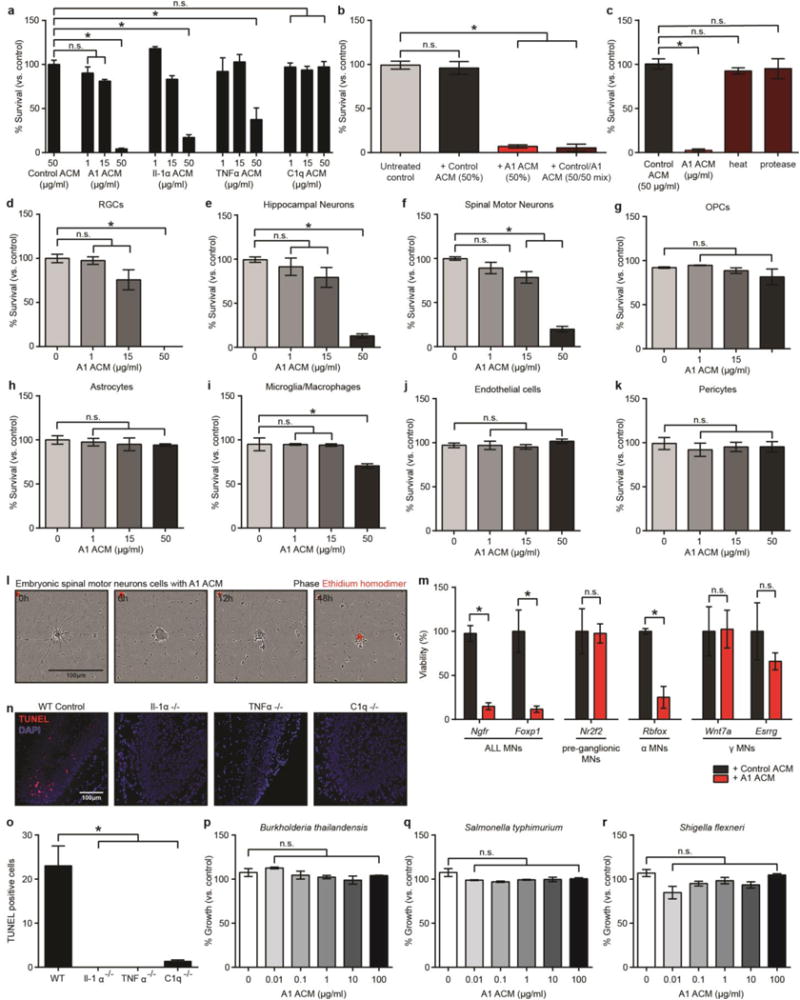
a, Quantification of dose-responsive cell death in retinal ganglion cells (RGCs) treated with astrocyte conditioned media from cells treated with Il-1α, TNFα, or C1q alone, or combination of all three (A1 astrocyte conditioned media, ACM) for 24 h. b, Death of RGCs was not due to a loss of trophic support, as treatment with 50% Control ACM did not decrease viability. Similarly, treatment with a 50/50 mix of Control and A1 ACM did not increase viability compared to A1 ACM only treated cells. c, A1-ACM-induced RGC toxicity could be removed by heat inactivation, or protease treatment. d–k, Cell viability of purified central nervous system cells treated with A1 ACM for 24 h: RGCs (d), hippocampal neurons (e), embryonic spinal motor neurons (f), oligodendrocyte precursor cells (OPCs, g), astrocytes (h), microglia/macrophages (i), endothelial cells (j), and pericytes (k). N = 4 for each experiment. l, Representative phase image showing death of purified embryonic spinal motor neurons in culture over 18 h (ethidium homodimer stain in red shows DNA in dead cells). m, qPCR for motor neuronal subtype-specific transcripts after 120h treatment with A1 ACM (50μg/ml). There was no decrease in levels of transcript for Nr2f2 (pre-ganglionic specific) and Wnt7a and Esrrg (γ specific), suggesting these motor neuron subtypes are immune to A1-induced toxicity. n, representative images with terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) staining in the dentate gyrus for wild type and Il1α−/−, TNFα−/−, or C1q−/− individual knockout animals following systemic LPS injection. Individual knock-out animals had far less TUNEL+ cells in the dentate gyrus (no cells in Il1α−/− or TNFα−/− animals) than wild type animals, suggesting A1-induced toxicity may be apoptosis. p–r, Percentage growth rate of gram negative bacterial cultures treated with A1 ACM for 16 h: B. thaliandensis (p), S. typhimurium (q), S. flexneri (r). N = 3. * p < 0.05, one-way ANOVA. Error bars indicate s.e.m.
