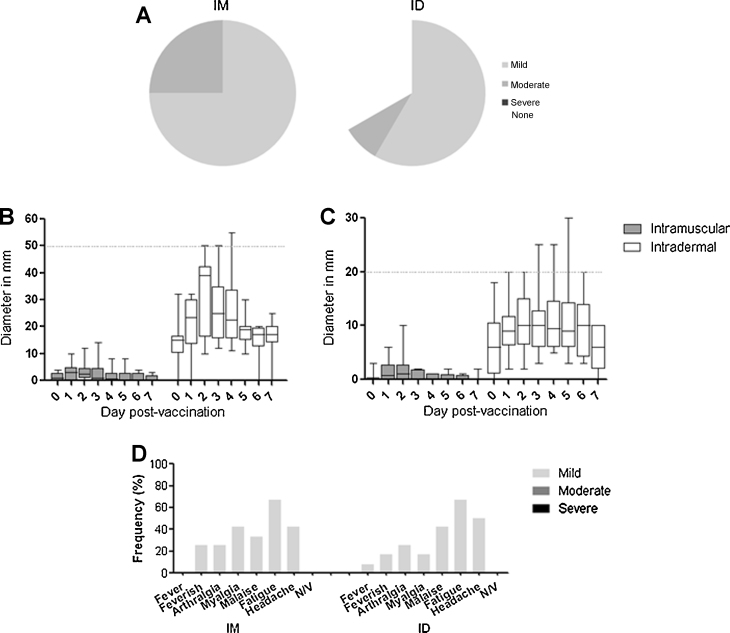
Fig. 2.
Local and systemic adverse events. (A) shows the proportion of subjects reporting mild, moderate, and severe local pain after intramuscular and intradermal vaccination. No subjects experienced severe pain. Subsequent figures show the diameter of erythema (B) and swelling (C) in mm at the injection site of subjects during the first 7 days after vaccination, and the frequency of systemic adverse events (D) reported by subjects during the first 7 days after vaccination. All data are shown by group. Box and whisker plots show median, interquartile range, and minimum and maximum values. The horizontal dashed line on (B) and (C) indicate the threshold between “mild” severity and “moderate” severity which is 50 mm for erythema and 20 mm for swelling. Only one subject experienced moderate erythema and swelling. N/V = nausea or vomiting.