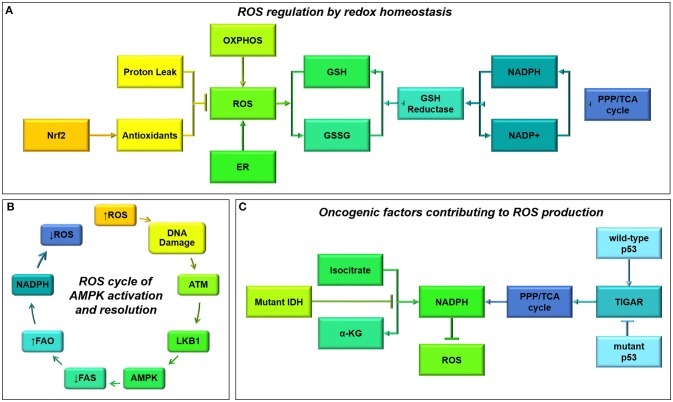
Figure 11.
(A) Reactive oxygen species (ROS) maintenance by redox homeostasis. Oxidative phosphorylation and endoplasmic reticulum (ER) activity both result in the formation of ROS (Salazar-Ramiro et al., 2016). These ROS can be neutralized by antioxidants, under the transcriptional control of Nrf2, the master regulator of ROS homeostasis (Cardaci and Ciriolo, 2012). Additionally, proton leak in the mitochondria also helps to neutralize ROS through a self-regulating system (Brookes, 2005). Another system to regulate ROS levels, acts through the glutathione (GSH) system which neutralizes ROS upon conversion into glutathione disulphide (GSSG) (Levine and Puzio-Kuter, 2010). Within cells, GSH levels are maintained by production of NADPH from the PPP and Kreb's cycle which maintain GSH reductase activity (Levine and Puzio-Kuter, 2010). (B) ROS cycle of AMPK activation and resolution. Increased ROS levels result in oxidative DNA damage and the activation of the DNA damage response pathway by ataxia-telangiectasia mutated (ATM) recognition (Alexander and Walker, 2011). This stimulates LKB1 activity, and increased phosphorylation and stimulation of AMPK. As a result AMPK inhibits NADPH consuming pathways such as FAS and activates catabolic NADPH-producing pathways such as FAO, which acts through the GSH/GSSG antioxidant system to neutralize ROS and restrain further oxidative damage (Jeon et al., 2012). (C) Oncogenic factors contributing to ROS production. Two common mutations in secondary gliomas include the mutation of isocitrate dehydrogenase (IDH) and p53 pathways (Cuperlovic-Culf et al., 2012). Through their downstream activities, i.e., IDH reducing isocitrate into α-KG and p53-mediated transcription of TIGAR, these proteins aid in maintaining NADPH levels and subsequent ROS neutralization (Wanka et al., 2012b; Klink et al., 2016). However, upon mutation these pathways are inhibited resulting in a loss of NADPH, increased ROS and a higher rate of oxidative damage within glioma cells.