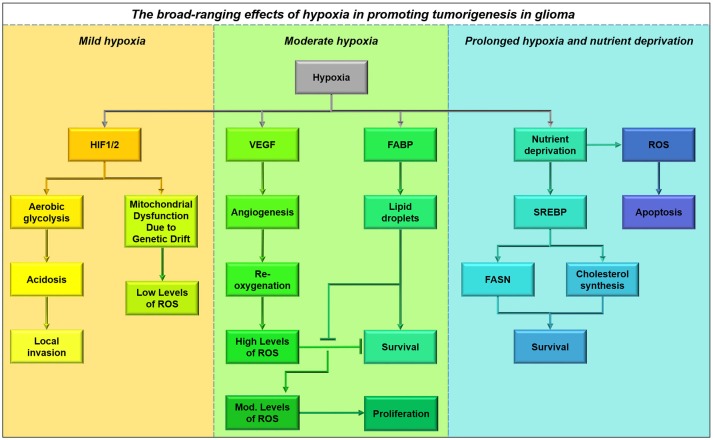
Figure 13.
The broad-ranging effects of hypoxia in promoting tumorigenesis in glioma, and protection from immediate ill effects of re-oxygenation. Acute periods of hypoxia result in the up-regulation of many glycolytic genes resulting in the release of lactate and an acidic microenvironment, which is conducive for localized invasion. Hypoxia is also accompanied by mitochondrial dysfunction as a result of this metabolic adaptation, resulting in excess accumulation of ROS and apoptosis. Under conditions of moderate hypoxia, GBM cells downregulate fatty acid, and cholesterol biosynthesis pathways and primarily rely on increased fatty acid uptake to meet their nutrient requirements, through upregulation of fatty acid binding proteins particularly FABP7, resulting in the formation of lipid droplets in a time- and oxygen-dependent manner (Bensaad et al., 2014). Restricted oxygen availability and nutrient deprivation are often accompanied by poor vascularisation, causing extensive increases in apoptosis (Lewis et al., 2015). However, under these conditions some glioma cells have been shown to upregulate sterol regulatory element-binding protein (SREBP) to maintain fatty acid and cholesterol metabolism by disrupting the mevalonate pathway (Lewis et al., 2015). The accumulation of lipids in GBM helps to maintain viability upon re-oxygenation after angiogenesis, providing an alternative source for ATP production and protecting against ROS accumulation (Bensaad et al., 2014).