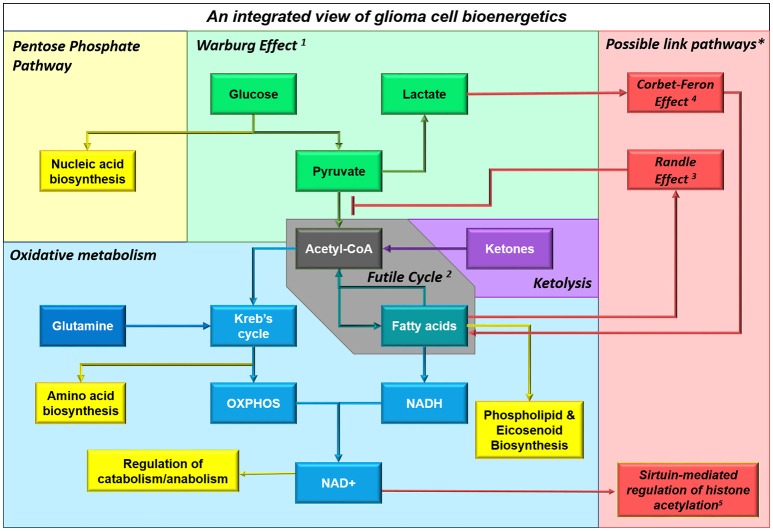
Figure 4.
Multiple substrates can contribute to cellular bioenergetics by providing acetyl CoA. This schematic shows an integrated view of glioma cell metabolism, demonstrating that ketones providing acetyl CoA will fuel both energy production and biosynthesis of raw materials (e.g., nucleic acids, amino acids, phospholipids and other molecules). The non-oxidative use of glucose by glioma cells is shown in green; particularly the Warburg Effect1 where cancer cells undergo glycolysis that is not followed by oxidation, instead converting the end product pyruvate into lactate and releasing it into the extracellular space. Aerobic respiratory pathways used by glioma cells are shown in blue; glioma cells appear to engage in a Futile Cycle2 (gray) where fatty acids are continuously synthesized and oxidized in glioma cells (it has been shown that inhibiting either pathway reduces cellular proliferation and slows tumor progression). Ketolysis, shown in violet, and fatty acid oxidation, shown in teal, provides acetyl CoA which fuels the Kreb's Cycle and oxidative phosphorylation; acetyl CoA produced from any source facilitates anapleurosis and biosynthesis of many molecules critical to cellular function. Metabolic intermediates, such as those produced by the Kreb's Cycle and oxidative phosphorylation, impinge on other cellular functions, as shown in yellow. *Interactions between enzymes that link catabolic pathways, which have been identified in other cancers but not yet studied in glioma, are shown in red. These include: the Randle Effect3 where NADH and acetyl CoA produced during fatty acid (FA) oxidation inhibit pyruvate dehydrogenase, thus promoting glucose's alternative fate (release as lactate), the Corbet-Feron Effect4 where lactate-induced acidification of the microenvironment promotes the FA oxidation phenotype via acetylation-mediated activation of mitochondrial proteins, and sirtuin-mediated regulation of histone acetylation5.