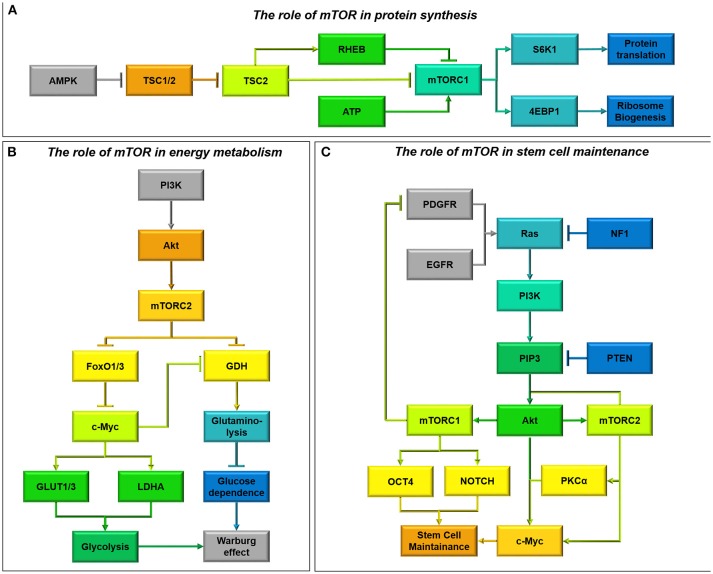
Figure 8.
(A) The role of mTOR in protein synthesis. AMPK is a well-known regulator of mTOR activity (Vucicevic et al., 2011). Upon activation AMPK destabilizes the Tumor Suppressor 1/2 (TSC1/2) complex, allowing TSC2 to freely bind Ras-homolog enriched in brain (RHEB) and inhibit mTORC1 signaling (Laplante and Sabatini, 2012). This means that energy consuming processes such as protein translation and ribsome biogenesis are inhibited during periods of energy stress. As well as acting through AMPK, ATP has recently also been shown to be able to directly activate mTORC1 signaling (Dennis et al., 2001). (B) The role of mTOR in energy metabolism. mTORC2 metabolic reprogramming downstream of PI3K/Akt is a critical regulator of the Warburg effect and glucose dependence in glioma cells (Masui et al., 2015). By relieving FoxO1/3 constraint on c-Myc signaling, mTORC2 increases Glucose Transproter 1 and 3 expression (GLUT1/3) as well as lactate dehydrogenase activity, encouraging aerobic glycolysis (Masui et al., 2013). Simultaneously, through c-Myc activity mTORC2 also inhibits glutamate dehydrogenase (GDH) activity required for glutaminolysis, regulating a metabolic switch from oxidative phosphorylation fuelled by the Kreb's cycle to aerobic glycolysis (Yang et al., 2009). mTORC2 signaling therefore causes glioma cells to become “addicted” to aerobic glycolysis, making them particularly vulnerable to glucose depletion. (C) The role of mTOR in stem cell maintenance. Oncogenic activity which converges on PI3K can activate mTOR signaling (Sarbassov et al., 2005). mTORC1 and mTORC2 activity results in the activation of several transcription factors, including OCT4 and NOTCH and c-Myc respectively, ensuring stem cell maintainence (Masui et al., 2013). c-Myc can also be activated directly by Akt signaling and PKCα signaling downstream of mTORC2 to induce stem cell transformation (Fan et al., 2009). Additionally, whilst mTORC2 activity forms part of a feed-forward system, phosphorylating Akt, mTORC1 activity downregulates PI3K activity by downregulating PDGFR (Akhavan et al., 2010; Sarbassov et al., 2005).