Figure 3.
apc15Δ Mutants Have Significant Defects in the Processivity of Cdc20Slp1 Ubiquitination, and Fission Yeast MCC-Cdc20-APC/C Contains Two Molecules of Cdc20
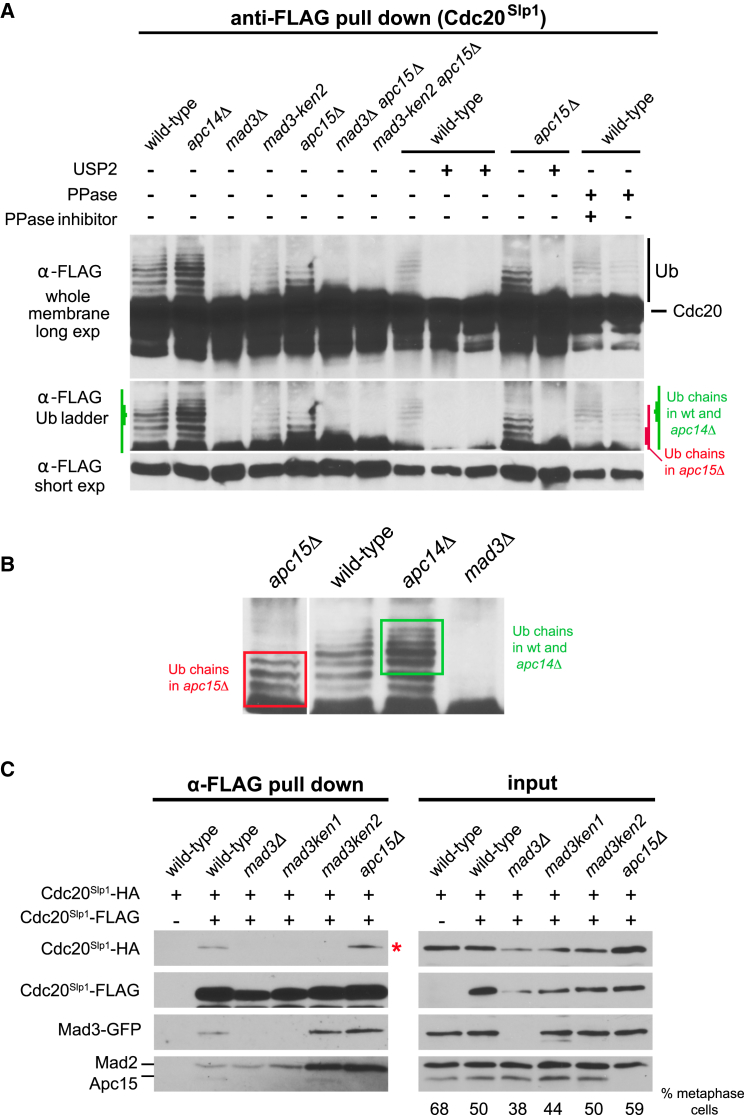
(A) Cdc20 ubiquitination experiments. The indicated strains all contain the mts3-1 proteasome mutation, to block cells in mitosis independent of the spindle checkpoint and to enrich for poly-ubiquitinated forms of cellular proteins. Cultures were shifted to 36°C 3 hr prior to harvesting. Whole-cell lysates were made in the presence of Dub inhibitors, and Cdc20-FLAG was immunoprecipitated and then immunoblotted for Cdc20-FLAG. Long exposure reveals a ladder of slow-migrating bands, which are reduced in mad3 and apc15 mutants. The indicated lysates were treated with recombinant hsUSP2 (de-ubiquitinase) or lambda phosphatase prior to running the gel. Lambda phosphatase has no effect but USP2 abolishes the ladder, confirming that this is due to modification with ubiquitin. Different modified forms of Cdc20 accumulate in the mts3 and apc15Δmts3 mutants, with shorter chains in the absence of Apc15. These are indicated with green and red markings by the relevant anti-ubiquitin blots. This experiment was repeated three times.
(B) The indicated lanes from (A) are expanded to highlight the different modified forms of Cdc20 that accumulate in the mts3, apc15Δmts3, and apc14Δmts3 mutants. Most notably, there are shorter chains in the absence of Apc15 (boxed in red).
(C) Cdc20-FLAG and Cdc20-HA co-immunoprecipitate. Cells containing both Cdc20 forms were synchronized in mitosis (60 min after cdc25 block and release), lysates were prepared, and Cdc20-FLAG was immunoprecipitated. The immunoprecipitates were then immunoblotted and analyzed for associated Cdc20-HA, Mad3-GFP, Mad2, and Apc15. The asterisk indicates Cdc20-Cdc20 co-immunoprecipitation. This experiment was repeated three times. The % of metaphase cells is indicated below the blot for the six strains.
See also Figure S3.