Highlights
-
•
Children living in these settings had a high prevalence of enteropathogens, high levels of intestinal inflammation, abnormal intestinal permeability, high markers of systemic inflammation, and postnatal acquired linear growth deficits when compared to children living in the US or Europe
-
•
This study contributes empiric evidence to demonstrate that enteric infection alters both fecal markers of inflammation and permeability
-
•
Current markers of enteropathy fail to account for a large portion of the observed shortfalls in linear growth in these populations, and markers of systemic inflammation appear as the most promising predictive biomarkers for identifying linear growth failure in children
Environmental enteropathy (EE) is hypothesized as a mediator of growth faltering, but few prospective studies have evaluated pathways linking enteropathogen exposure, intestinal inflammation and permeability, and growth. The MAL-ED study represents a novel analytical framework and explicitly evaluates multiple putative EE pathways in combination and using an unprecedented quantity of data. Despite evidence that gut inflammation and altered gut permeability are frequently present and that associations between enteropathogen exposure and gut dysfunction exist, the observed attributable effects of EE on growth faltering in young children were small.
Keywords: Enteropathy, Undernutrition, Stunting, Enteropathogen, Child growth, Child health
Abstract
Background
Environmental enteropathy (EE), the adverse impact of frequent and numerous enteric infections on the gut resulting in a state of persistent immune activation and altered permeability, has been proposed as a key determinant of growth failure in children in low- and middle-income populations. A theory-driven systems model to critically evaluate pathways through which enteropathogens, gut permeability, and intestinal and systemic inflammation affect child growth was conducted within the framework of the Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development (MAL-ED) birth cohort study that included children from eight countries.
Methods
Non-diarrheal stool samples (N = 22,846) from 1253 children from multiple sites were evaluated for a panel of 40 enteropathogens and fecal concentrations of myeloperoxidase, alpha-1-antitrypsin, and neopterin. Among these same children, urinary lactulose:mannitol (L:M) (N = 6363) and plasma alpha-1-acid glycoprotein (AGP) (N = 2797) were also measured. The temporal sampling design was used to create a directed acyclic graph of proposed mechanistic pathways between enteropathogen detection in non-diarrheal stools, biomarkers of intestinal permeability and inflammation, systemic inflammation and change in length- and weight- for age in children 0–2 years of age.
Findings
Children in these populations had frequent enteric infections and high levels of both intestinal and systemic inflammation. Higher burdens of enteropathogens, especially those categorized as being enteroinvasive or causing mucosal disruption, were associated with elevated biomarker concentrations of gut and systemic inflammation and, via these associations, indirectly associated with both reduced linear and ponderal growth. Evidence for the association with reduced linear growth was stronger for systemic inflammation than for gut inflammation; the opposite was true of reduced ponderal growth. Although Giardia was associated with reduced growth, the association was not mediated by any of the biomarkers evaluated.
Interpretation
The large quantity of empirical evidence contributing to this analysis supports the conceptual model of EE. The effects of EE on growth faltering in young children were small, but multiple mechanistic pathways underlying the attribution of growth failure to asymptomatic enteric infections had statistical support in the analysis. The strongest evidence for EE was the association between enteropathogens and linear growth mediated through systemic inflammation.
Funding
Bill & Melinda Gates Foundation.
1. Introduction
Traditionally, the evaluation of pathogenicity from enteric infections in the host has focused on the evaluation of defined diarrheal or acute gastrointestinal illnesses and the health outcomes associated with such illness perceived as binary, that is, either death or survival. It has been posited that the host response to frequent enteric infections alters the gut in a way that adversely affects the health status of the host even in the absence of diarrhea or acute gastrointestinal illness. The consequences of this altered host phenotype may have long term effects on child health and development potential. This condition is known as environmental enteropathy (EE). A proposed consequence of EE is reduced linear growth in children (Lunn, 2000, Keusch et al., 2013, Keusch et al., 2014), and EE may explain the less than expected effectiveness of nutritional interventions to improve growth in developing populations (Lunn et al., 1991, Humphrey, 2009, Korpe and Petri, 2012, Kosek et al., 2014). Several mechanisms have been proposed to explain how EE results in poorer nutritional status by reducing functional capacities of the gut. These include reduced absorptive capacity (Kelly et al., 2004, Menzies et al., 1999), increased permeability (Lunn et al., 1991), and chronic intestinal and systemic inflammation with resulting metabolic changes that affect nutrient and micronutrient availability and utilization (Campbell et al., 2003, Kosek et al., 2013).
Multiple physiological mechanisms by which enteropathogens can disrupt gut functioning have been identified (Guerrant et al., 1999, Berkes et al., 2003, Beltinger et al., 2008, Viswanathan et al., 2009, Kamada et al., 2013, Brown et al., 2015) although the long term consequences in settings where exposure to enteropathogens is intense and continuous (Platts-Mills et al., 2015) are poorly understood. Populations in low- and middle-income countries are also subject to other causes of growth failure, including inadequate dietary intake and frequent overt illness, any of which may influence both EE biomarkers and observed growth outcomes.
The collection of non-invasive biomarkers of EE is expanding, with different markers characterizing different aspects of gut physiology and integrity. The most widely used EE biomarker is the lactulose:mannitol (L:M) dual sugar test for intestinal permeability (Menzies et al., 1999, Denno et al., 2014), which has been used to demonstrate that altered gut permeability is related to the risk of stunting and is prevalent in environments with poor sanitation (Lunn et al., 1991, Lin et al., 2013, Weisz et al., 2012). Other EE bioassays available include fecal markers of gut inflammation (Campbell et al., 2004), intestinal growth factors (Peterson et al., 2013), and plasma markers of bacterial translocation (Naylor et al., 2015). Additionally, an increasing set of markers are becoming available that encompass systemic inflammation and amino acid and lipid metabolism (Campbell et al., 2003, Mondal et al., 2012, Hashimoto et al., 2012, Mayneris-Perxachs et al., 2016, Semba et al., 2016). Aligning the pathways indicted by this expanding collection of biomarkers with enteric infections and growth in early infancy and childhood across different populations is the subject of considerable current effort (Kosek et al., 2013, Peterson et al., 2013, Prendergast and Kelly, 2012).
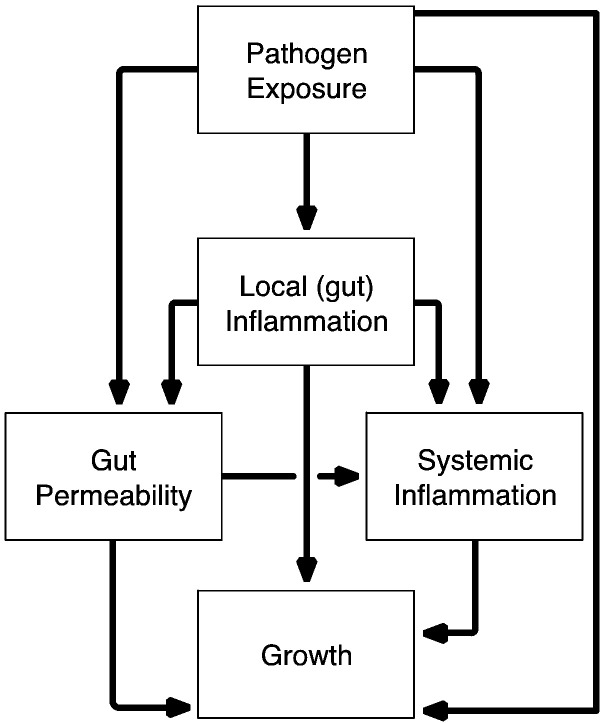
The Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development (MAL-ED) study was designed to assess the role of enteropathogens and other factors in growth faltering from birth to two years across eight sites (MAL-ED Network Investigators, 2014a). A central hypothesis of MAL-ED is that gut injury resulting in disruption of normal physiology is the key route by which enteropathogens contribute to malnutrition. Here, we use a causal systems model (a directed acyclic graph [DAG]) to test key theoretical pathways of the EE conceptual model and examine how enteropathogen infection results in impaired physical growth in infancy and early childhood (Fig. 1).
Fig. 1.
Conceptual model of the associations between pathogens, markers of gut function and inflammation, systemic inflammation and growth.
2. Methods
2.1. Study Design and Population
The MAL-ED study, conducted in eight diverse sites on three continents: Bangladesh (Dhaka: BGD), India (Vellore: INV), Nepal (Bhaktapur: NEB), and Pakistan (Naushero Feroze: PKN) in Southern Asia; Brazil (Fortaleza: BRF) and Peru (Loreto: PEL) in Latin America; and South Africa (Venda: SAV) and Tanzania (Haydom: TZH) in Sub-Saharan Africa. The study design is described in detail elsewhere (MAL-ED Network Investigators, 2014b). In brief, children were enrolled within 17 days of birth, but excluded if they had a birth weight < 1500 g, were very ill, or were non-singleton; or if their mother was < 16 years of age. Approximately 10 children were enrolled per month per site, with the goal of retaining ≥ 200 per site at 24 months of age (after loss to follow up). Data collection methods are described elsewhere for illness and treatment (Richard et al., 2014), infant feeding (Caulfield et al., 2014), and stool microbiology (Houpt et al., 2014). Each site obtained ethical approval from their respective institutions and written consent was obtained from participants.
Non-diarrheal stool samples collected monthly in the first year and quarterly in the second year were evaluated for > 40 pathogens using a standardized approach (Houpt et al., 2014). In addition to analyzing total number of pathogens detected per stool, we also categorized pathogens into five groups based on pathophysiology. Group I included viruses that cause limited mucosal disturbances (rotavirus, adenovirus and astrovirus). Group II included bacteria that are enteroinvasive or cause extensive mucosal disruption (Campylobacter, Shigella, Salmonella, Plesiomonas, Yersinia, enteroaggregative E. coli (EAEC), enteropathogenic E. coli (EPEC), enteroinvasive E. coli (EIEC) and Aeromonas). Group III was enterotoxigenic E. coli (ETEC), which is a cause of secretory diarrhea with only limited mucosal changes. Cryptosporidium (Group IV) and Giardia (Group V) were considered independently as organisms have both been shown to be associated with linear growth failure and prolonged and persistent carriage.
Three fecal biomarkers relating to aspects of gut inflammation and immunity (“local inflammation” in Fig. 1) were evaluated using the same non-diarrheal stool samples assayed for enteropathogens:(Kosek et al., 2014, Kosek et al., 2013) (1) myeloperoxidase (MPO, ng/mL) as a marker of neutrophil activity in the intestinal mucosa (Alpco, Salem, NH, USA); (Keusch et al., 2013) neopterin (NEO, nmol/L) to indicate T-helper cell 1 activity (GenWay Biotech, San Diego, CA, USA); and (Keusch et al., 2014) alpha-1-antitrypsin (AAT, mg/g) to indicate protein loss and intestinal permeability (Biovendor, Candler, NC, USA). Because diarrhea leads to stool dilution, fecal biomarker values were excluded if proximate to diarrheal symptoms (within seven days prior). Similarly, stools collected the day of or the day following the L:M test were excluded as this test is an osmotic laxative.
In addition to fecal biomarkers, urinary L:M testing (“gut permeability” in Fig. 1) was performed at three, six, nine, and 15 months, as described elsewhere (Kosek et al., 2014). Urine samples were processed using high-performance liquid chromatography and pulsed amperometric detection or ion chromatography (depending on study site). The results were converted into a sample-based Z-score (LMZ) to minimize age and sex trends. Data from the BRF cohort were used as the internal reference standard.
Finally, systemic inflammation was evaluated at seven, 15, and 24 months using alpha-1-acid glycoprotein (AGP) concentration in plasma. Incidence of acute lower respiratory infection (ALRI), diarrhea, fever (associated with neither ALRI nor diarrhea), and a composite for any of the three categorized illness episodes in the seven or 14 days preceding the blood collection were drawn from bi-weekly maternal reports. These were used to examine the influence of recent, non-diarrheal, overt illness on AGP concentration.
Monthly length (cm) and weight (kg) measures (Lohmann et al., 1988) were converted to Z-scores (LAZ, WAZ respectively) based on WHO 2006 standards (World Health Organization, 2006). The change (Δ) in LAZ and WAZ for each child (final minus initial value for each period) served as the outcome in all analyses, controlling for the initial value. Intense quality assurance review procedures identified bias within the PKN length measures; therefore, these data were excluded from the system analysis. PKN biomarker data were however, included in the evaluation of associations between pathogens and biomarkers.
2.2. Statistical Analysis
First, to maximally leverage the large size of the MAL-ED dataset and to place our results in the context of previous studies, we analyzed relationships between pathogens and fecal biomarker concentrations, between pathogens and LMZ scores, between LMZ scores and changes in anthropometry, and among potential sources of systemic inflammation not associated with gut enteropathy.
Linear mixed effects models were constructed to examine cross-sectional associations between individual pathogens and concentrations of each fecal biomarker. Specifically, the log concentrations of MPO, NEO, and AAT were modeled as functions of stool consistency (a categorical description of stool liquidity), linear and quadratic terms for child age (to capture age-related trends), the presence of individual pathogens (adjusting for the presence of other pathogens), and a random intercept for child nested in site (McCormick et al., 2016).
The same model structure was extended to evaluate associations between pathogen presence and LMZ scores, limiting the analyses to non-diarrheal stools collected at the same age as the L:M test. Additionally, changes in anthropometry (ΔLAZ and ΔWAZ) over three, six, and nine month windows starting at each L:M assay were evaluated as a function of the LMZ scores. Individual children nested within their respective site were treated as a random intercept to account for clustering at both the individual child and site levels.
To determine whether the concentration of AGP was related to overt illness in the seven or 14 days preceding blood collection, another linear mixed effects model was constructed with log-transformed AGP concentration as a function of age and illness (i.e., the presence of diarrhea, fever, and ALRI). A random intercept for child nested in site was included.
In addition to these linear regressions and given that disease systems composed of different interacting pathways lend themselves to causal graphical modeling (e.g., Fig. 1) (Pearl, 1995, Greenland et al., 1999) we constructed a DAG model to test hypothetical pathways between the presence of enteropathogens, biomarker concentrations, and changes in LAZ and WAZ. Combining all factors into a single system allowed for the explicit partition of associations into direct and indirect pathways.
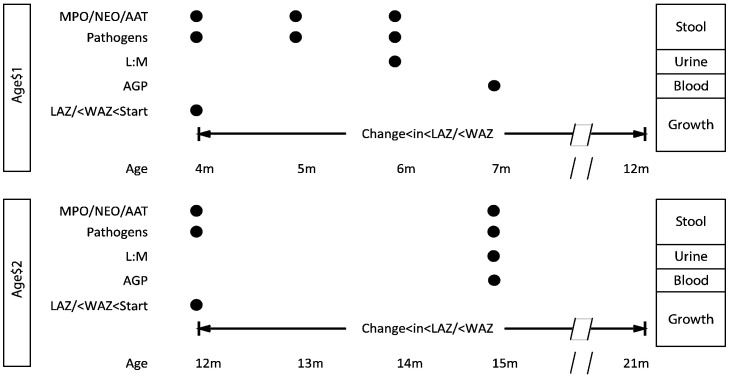
Variables within this system were represented as conditionally independent, multivariate, generalized linear mixed models such that the probability of observing a given value for each variable was a function of other variables connected within the system (indicated by arrows in Fig. 1). To account for heterogeneity between sites, random effects for both site and child were added at every node. The DAG analysis focused on two time periods, 4 ≤ months ≤ 11 (Age 1) and 12 ≤ months ≤ 21 (Age 2), using the data collection schedule shown in Fig. 2 to capture temporal associations between events marked by the biomarkers. Specifically, pathogen data was coincident with collection of the fecal biomarkers (MPO, NEO, and AAT). Their collection preceded collection of L:M as alterations in gut permeability are a hypothesized result of inflammation. Measures of systemic inflammation then followed. The temporal window then extended beyond biomarker collection to assess associations with subsequent growth.
Fig. 2.
Timeline for collection of stool, urine, and blood samples and their respective biomarker assays that relate to changes in growth Z-scores.
The net effects of both direct and indirect pathways were simulated from the fitted DAG. Sensitivities of ΔLAZ and ΔWAZ to changes in each biomarker were examined by fixing each biomarker to its observed mean concentration as well as one standard deviation higher or lower. The ΔLAZ and ΔWAZ were then simulated and the difference between their mean values when biomarkers were raised or lowered relative to when they were held at mean concentration were estimated.
The model was run in JAGS (version 3.4.0) to perform Markov Chain Monte Carlo simulations (Plummer, 2003). Further details are given in the Appendix.
2.3. Role of the Funding Source
The Bill & Melinda Gates Foundation did not play any role in the writing of the manuscript nor did the funders have of the study had any role in the study design, data collection, analysis, or interpretation of study results. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
Data from the entire cohort were included in the linear analyses for associations between pathogens and fecal biomarkers and LMZ, the changes in anthropometry following the L:M test, and between illness and AGP (Table 1, Table 2). Among the over 20,000 non-diarrheal stools tested for concentrations of MPO, NEO, and AAT, there was a trend for the concentration of each biomarker to decrease with increasing age. Within individual children; however, biomarker concentrations were highly variable across time, with intra-class correlations (ICC) of 0·07, 0·03, and 0·06 for MPO, NEO, and AAT respectively. Pearson correlation coefficients between the biomarkers were low (≤ 0·2) suggesting they measured different physiological insults.
Table 1.
The number of samples collected and with complete data to yield observations in individual children included in the system model. The analysis of individual biomarkers includes all available observations.
| Age 1 (4 ≤ m ≤ 11) |
Age 2 (12 ≤ m ≤ 21) |
||||
|---|---|---|---|---|---|
| Collected | Complete | Collected | Complete | ||
| Samples | Blood | 1503 | 1476 | 1536 | 1503 |
| Urine | 1767 | 1601 | 1706 | 1535 | |
| Non-diarrheal stools | 4481 | 4285 | 2821 | 2676 | |
| Anthropometry | 15,272 | 15,257 | 16,232 | 16,215 | |
| Children | Total | 2001 | 1873 | ||
| Excluding PKN | 1734 | 1617 | |||
| Complete data | 1059 | 1070 | |||
Table 2.
Observed characteristics of the subset of the MAL-ED population with complete data that were included in the system model. The mean and standard deviation (SD) of the continuous variables (anthropometry and the biomarkers) are shown along with the percentage of the discrete variables that were positive for at least one pathogen in each of the groups or the presence of maternally reported symptoms of ALRI and fever preceding the AGP assay and the percentage of stools that were coincident with different food intakes.
| Continuous variables | Age 1 |
Age 2 |
Discrete variables | Age 1 | Age 2 | |||
|---|---|---|---|---|---|---|---|---|
| Mean | ± SD | Mean | ± SD | % Positive | % Positive | |||
| LAZ start | − 0.97 | 1.13 | − 1.05 | 1.05 | Pathogens | Group 1 | 6.85 | 9.11 |
| ΔLAZ | − 0.06 | 0.63 | − 0.20 | 0.47 | Group 2 | 56.76 | 65.86 | |
| WAZ start | − 0.53 | 1.15 | − 0.61 | 1.21 | Group 3 | 6.85 | 10.21 | |
| ΔWAZ | − 0.04 | 0.50 | − 0.13 | 0.41 | Group 4 | 3.43 | 5.10 | |
| log(MPO) | 8.90 | 1.26 | 8.33 | 1.27 | Group 5 | 3.36 | 18.35 | |
| log(NEO) | 7.69 | 1.08 | 7.30 | 1.30 | ALRI | 1.71 | 1.94 | |
| log(AAT) | − 0.89 | 0.93 | − 1.17 | 1.13 | Fever | 28.26 | 24.18 | |
| log(AGP) | 4.61 | 0.40 | 4.63 | 0.36 | ||||
| LMZ | 0.35 | 0.87 | 0.40 | 1.12 | ||||
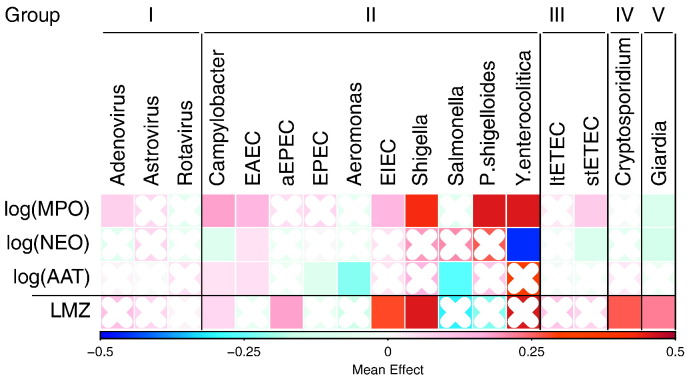
A comparison of associations between enteropathogens and the fecal biomarkers revealed that pathophysiological groups tended to show similar trends, with some prevalent pathogens being associated with either higher or lower biomarker concentrations (e.g., Campylobacter and EAEC were associated with higher concentrations of MPO and AAT, while Giardia was associated with lower concentrations of all three fecal biomarkers). The associations were more pronounced in some of the rarer pathogens (e.g., Yersinia enterocolitica was strongly associated with increased MPO and decreased NEO concentrations the few times it was detected) (Fig. 3). These exploratory models assumed additive effects of pathogens, though in many instances more than one pathogen was detected.
Fig. 3.
Effect of pathogens on the three fecal biomarkers (N non-diarrheal stools = 27,931) and the L:M test (N urine samples = 4476). The color represents the coefficient from a linear mixed effects model with pathogens found in the same stool as the fecal biomarkers or during the same month as the L:M test. Cells with crosses are not significant (p ≥ 0.05). Pathogens, within their groups (I˗V), are sorted by prevalence (high to low, left to right). In addition to the presence of individual pathogens, age was included using both linear and quadratic terms, stool consistency was included in the biomarker models, and child nested in site was included as a random intercept.
LMZ tended to be higher, indicating increased permeability, in children with pathogens detected, especially those with positive tests for Cryptosporidium (0·34 ± 0·08 for mean ± standard deviation, N = 134) and Giardia (0·20 ± 0·05, N = 457) (Fig. 3). However, there was no statistical support for LMZ relating to changes in either LAZ or WAZ (Table 3).
Table 3.
The relationship between the LMZ and changes in LAZ and WAZ over a three, six, or nine month period from the age of the L:M test. L:M tests were performed at three, six, nine, and 15 months. Child nested in study site was considered a random intercept to account for repeated measures and site heterogeneity.
| Mean effect (± standard error) |
||||||
|---|---|---|---|---|---|---|
| ΔLAZ (months post L:M test) |
ΔWAZ (months post L:M test) |
|||||
| 3 m | 6 m | 9 m | 3 m | 6 m | 9 m | |
| Constant (3 m L:M) | − 0.21 (0.08)⁎, ⁎⁎ | − 0.71 (0.20)⁎⁎⁎ | − 1.08 (0.25)⁎⁎⁎ | − 0.21 (0.08)⁎ | − 0.71 (0.20)⁎⁎⁎ | − 1.08 (0.25)⁎⁎⁎ |
| Constant (6 m L:M) | − 0.12 (0.02)⁎⁎⁎ | − 0.21 (0.02)⁎⁎⁎ | − 0.19 (0.02)⁎⁎⁎ | − 0.12 (0.02)⁎⁎⁎ | − 0.21 (0.02)⁎⁎⁎ | − 0.19 (0.02)⁎⁎⁎ |
| Constant (9 m L:M) | − 0.24 (0.02)⁎⁎⁎ | − 0.33 (0.02)⁎⁎⁎ | − 0.27 (0.02)⁎⁎⁎ | − 0.24 (0.02)⁎⁎⁎ | − 0.33 (0.02)⁎⁎⁎ | − 0.27 (0.02)⁎⁎⁎ |
| Constant (15 m L:M) | − 0.25 (0.02)⁎⁎⁎ | − 0.40 (0.02)⁎⁎⁎ | − 0.33 (0.02)⁎⁎⁎ | − 0.25 (0.02)⁎⁎⁎ | − 0.40 (0.02)⁎⁎⁎ | − 0.33 (0.02)⁎⁎⁎ |
| LAZ/WAZ at start | − 0.24 (0.01)⁎⁎⁎ | − 0.65 (0.01)⁎⁎⁎ | − 0.82 (0.01)⁎⁎⁎ | − 0.24 (0.01)⁎⁎⁎ | − 0.65 (0.01)⁎⁎⁎ | − 0.82 (0.01)⁎⁎⁎ |
| L:M Z-score (3 m) | 0.00 (0.02) | − 0.01 (0.01) | 0.00 (0.01) | 0.00 (0.02) | − 0.01 (0.01) | 0.00 (0.01) |
| L:M Z-score (6 m) | − 0.03 (0.02) | 0.03 (0.02) | − 0.01 (0.02) | − 0.03 (0.02) | 0.03 (0.02) | − 0.01 (0.02) |
| L:M Z-score (9 m) | − 0.01 (0.02) | − 0.00 (0.02) | − 0.02 (0.02) | − 0.01 (0.02) | − 0.00 (0.02) | − 0.02 (0.02) |
| L:M Z-score (15 m) | − 0.02 (0.02) | 0.00 (0.02) | 0.00 (0.02) | − 0.02 (0.02) | 0.00 (0.02) | 0.00 (0.02) |
| N | 5343 | 5213 | 5117 | 5343 | 5213 | 5117 |
| N children | 1650 | 1591 | 1563 | 1650 | 1591 | 1563 |
| N sites | 7 | 7 | 7 | 7 | 7 | 7 |
| Variance (child) | 0.03 | 0.37 | 0.61 | 0.03 | 0.37 | 0.61 |
| Variance (site) | 0.05 | 0.27 | 0.44 | 0.05 | 0.27 | 0.44 |
| Variance (Residual) | 0.29 | 0.18 | 0.13 | 0.29 | 0.18 | 0.13 |
p < 0·001.
p < 0·01.
p < 0·05.
With respect to systemic inflammation, approximately half the measures of AGP were elevated (> 100 mg/dL), indicating common subclinical inflammation. Across the two time points when AGP was measured, high concentrations were dispersed between children rather than focused in an identifiable subset (ICC 0·16). Across 4257 AGP assays, neither symptoms of ALRI (which was uncommon at the time of the two blood draws) nor diarrhea were associated with concentrations of AGP (Table 4). Maternally-reported fever in the 14 days prior to the blood draw was associated with increased AGP concentration. Only the subset of MAL-ED children with complete data (i.e. with no missing observations across all variables) was included in the DAG (Table 1).
Table 4.
The impact of recent overt symptoms on the log concentration of AGP based on samples at seven, 15, and 24 months. A linear mixed model of log(AGP) as a function of the presence or absence of overt symptoms in the 14 days preceding the blood draw. Child nested in site was treated as a random intercept.
| Variable | Mean (± standard error) |
|---|---|
| Constant | 4.58 (0.07)⁎⁎⁎, ⁎⁎, ⁎ |
| Diarrhea | 0.04 (0.05) |
| Fever | 0.21 (0.04)⁎⁎⁎ |
| ALRI | − 0.14 (0.07) |
| Age (months) | − 0.00 (0.00) |
| Diarrhea ∗ age | 0.00 (0.00) |
| Fever ∗ age | 0.00 (0.00) |
| ALRI ∗ age | 0.02 (0.00)⁎⁎⁎ |
| N | 4257 |
| N children | 1801 |
| N sites | 8 |
| Variance (child) | 0.01 |
| Variance (site) | 0.03 |
| Variance (residual) | 0.15 |
p < 0·001.
p < 0·01.
p < 0·05.
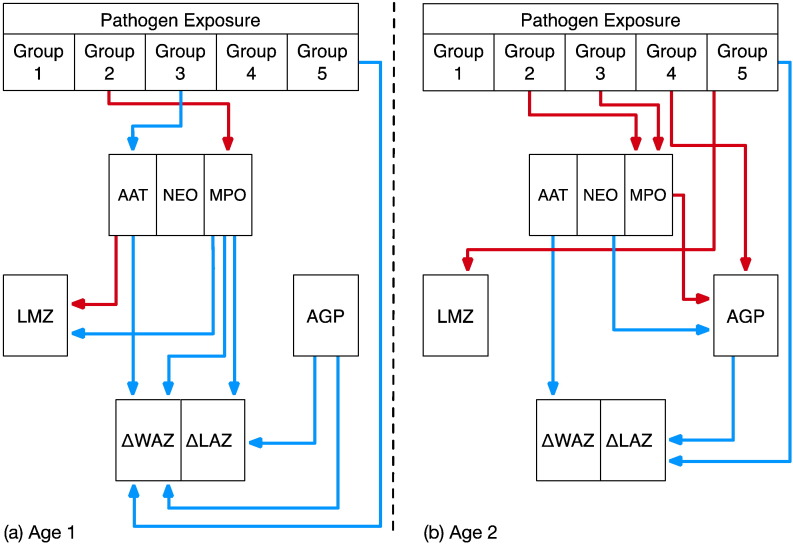
Within the systems model, which included the pathogen groups, all biomarkers, and both growth outcomes, the invasive bacteria (Group II) showed positive associations with MPO for both ages (Fig. 4). Additionally, ETEC (Group III) infection was associated with lower concentrations of AAT at Age 1 and higher concentrations of MPO in older Age 2 children. At Age 1, AAT concentration was positively associated with LMZ, while MPO was negatively associated with LMZ (Fig. 4). In the older age period, MPO concentration was positively associated with AGP, while NEO was negatively associated with AGP.
Fig. 4.
The model results for (a) Age 1 (4 ≤ months ≤ 11) and (b) Age 2 (12 ≤ months ≤ 21) using functional pathogen groupings and the specific pathways indicated by the individual fecal biomarkers as well as LMZ and AGP. Arrows show those relationships that had statistical support based on the 95% credibility interval. Red arrows indicate positive associations and blue arrows show negative associations. The pathogen groups reflect 1) viruses; 2) invasive bacteria; 3) non-invasive bacteria; 4) Cryptosporidium; and 5) Giardia.
Regarding associations between biomarkers and the two growth outcomes, ΔLAZ and ΔWAZ, the systems model revealed direct negative relationships between MPO and both ΔLAZ and ΔWAZ during Age 1 and negative associations between AAT and ΔWAZ in both age groups (Fig. 4) (i.e., the higher the concentration of MPO or AAT, the more restricted the growth over the respective period). There was no statistical support for an association between LMZ and either ΔLAZ or ΔWAZ in the system model (or for lactulose excretion, data not shown). Higher AGP concentrations were associated with decreased ΔLAZ at both ages and with ΔWAZ during Age 1 (Fig. 4). Because enteropathogens, particularly those associated with enteroinvasion or mucosal disruption (Group II), were directly related to increased MPO, they were thereby indirectly related to reduced growth. Giardia was associated directly with reduced growth, but not with the fecal biomarkers.
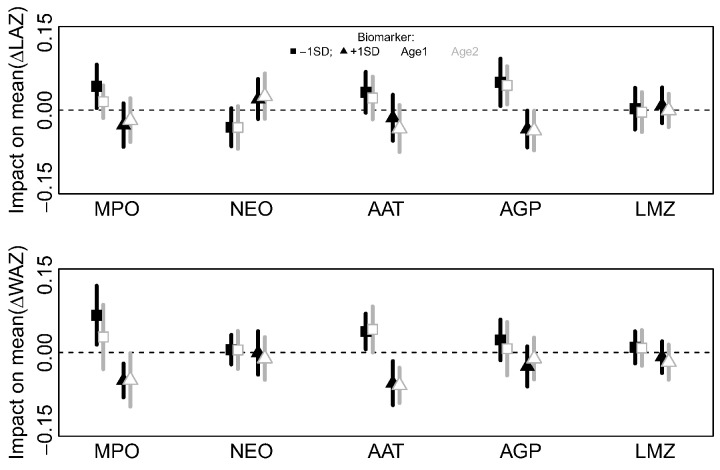
The effects of higher or lower biomarker concentrations relative to their observed means on ΔLAZ and ΔWAZ over each age period are shown in Fig. 5. None of the biomarkers had large mean effects on the average ΔLAZ or ΔWAZ. Of the effects on ΔLAZ, changing the log(AGP) concentration by ± 1SD produced the greatest difference (± 0·05 mean ΔLAZ), but little effect on the mean ΔWAZ (± 0·02 ΔWAZ) relative to the observed concentration of log(AGP). Changing the MPO concentration by ± 1SD had the second largest impact on ΔLAZ (+ 0·04 or − 0·03 for a decrease or increase in log(MPO) concentration respectively). The effect of increasing MPO concentration on ΔWAZ was marginally larger (− 0·05 ± 0·03), after which AAT had the second biggest impact on ΔWAZ (+ 0·04 when decreased and − 0·06 when log(AAT) was increased by 1SD).
Fig. 5.
Sensitivity analysis of the DAG model (Fig. 4) to explore the effect of increasing (triangles) or decreasing (squares) the concentration of different biomarkers on mean ΔLAZ and ΔWAZ at the two age periods (Age 1, black; Age 2, gray). Symbols indicate the mean difference (lines, ± 1 standard deviation) in the mean simulated ΔLAZ and ΔWAZ when biomarkers are changed ± 1 standard deviation compared to a simulation using the mean observed biomarker value (i.e., the dotted horizontal line shows a difference of zero).
4. Discussion
The systems model presented here is the first effort to explicitly combine enteropathogen exposure and different EE biomarkers (i.e., for gut permeability, gut inflammation, and systemic inflammation) into a single system and examine prospectively the pathways through which they relate to growth, thus providing an important proof of principal for a poorly defined condition. The temporal nature of the data allows for the exploration of causality and for the natural history of the biologic processes being measured to be interpreted with rigor. Our model is informed by a substantial quantity of structured longitudinal data on enteropathogens in non-diarrheal stools, illness history, intestinal inflammation and permeability, systemic inflammation, and growth across seven populations. The number of repeated measures over time and measures within the same individual is a particular strength of these data. Enteropathogen presence changes the concentration of contemporary biomarkers of gut immunity (NEO), inflammation (MPO), and permeability (AAT and LMZ). We find additional evidence that both enteropathogens and gut inflammation relate to systemic inflammation.
Our results yield several important findings. First, children living in these eight countries with differing epidemiologic settings have consistently and strikingly high concentrations of MPO (nearly 5–10 times the those seen in the USA) (Saiki, 1998) and nearly twice the concentration of AAT (Meyers et al., 1985). They also have notable elevations in AGP and intestinal permeability (L:M) that are two to three times higher than values in healthy populations (Kremer et al., 1988). Additionally, there was a high burden of enteropathogens detected even in the absence of overt diarrhea episodes (Platts-Mills et al., 2015). But most importantly, related MAL-ED analyses have demonstrated that, in the diverse epidemiologic settings of the study, growth velocities were low (MAL-ED Network Investigators, n.d.-a) leading to an increased prevalence of stunting by two years (MAL-ED Network Investigators, n.d.-b). The evidence we present here suggests that EE as measured by these markers contributes to, but does not appear to be the predominant driver of growth faltering.
As hypothesized, pathogen presence in non-diarrheal stool samples was associated with higher fecal MPO; this finding was also noted for AAT, although the AAT findings were evident only in younger children (aged 4˗11 months). When fecal biomarkers were evaluated for associations with individual pathogens, stronger signals of inflammation were noted in response to the rarely detected Shigella and Yersinia and the ubiquitous Campylobacter. The differential importance of pathogens in inducing EE is an area that requires additional attention, especially given the availability of newer technologies that allow for broad coverage and quantification of enteropathogens (Platts-Mills et al., 2015), and other strategies for determining which microbial taxa are inducing mucosal response in the gut (MAL-ED Network Investigators, n.d.-b). Such studies will enhance our understanding of the possible impact of etiology-specific interventions on EE.
Each of the three fecal biomarkers in this analysis have previously been associated with an elevated risk of stunting in the first year of life (George et al., 2015). Similar results have been found in other studies (Lin et al., 2013, Naylor et al., 2015). Here, we also find support for direct effects of gut inflammation (i.e., as measured by the three fecal biomarkers) on ΔLAZ or ΔWAZ. The evidence for an effect of systemic inflammation (i.e., as measured by AGP) is more compelling than that of the EE biomarkers. We found no evidence for an association between augmented intestinal permeability (i.e., as measured by LMZ) and future growth. Despite the popular use of L:M as a test of EE in cross sectional studies, its predictive power for future growth is less clear. While many studies have shown the L:M ratio to be associated with the contemporaneous LAZ (Lunn et al., 1991, Goto et al., 2009, Campbell et al., 2002), others have found (as we did) that it was not predictive of change in LAZ of children (Lin et al., 2013, Weisz et al., 2012). Given that AAT was associated with both gut permeability and subsequently to changes in growth (especially WAZ, both Age 1 and 2), it is possible that AAT more reliably reflects changes (or more severe changes) in permeability than the L:M test. The polar surface area of AAT greatly exceeds that of lactulose, and the size of the permeability defect may be important to delineate in greater detail using alternate probes. It is worth noting that other MAL-ED data (i.e., non-growth data) indicate the L:M test is predictive of impaired efficacy of oral polio vaccine (MAL-ED Network Investigators, n.d.-c). As such, the L:M test may capture other domains of EE not examined in these analyses.
Our results suggest that reductions in the exposure to pathogens, in particular to invasive bacteria that increase MPO, could reduce systemic inflammation (AGP). Recent evidence from another cohort study similarly highlighted the importance of systemic inflammation (Naylor et al., 2015). AGP interacts with bacterial lipopolysaccharide (LPS) (Moore et al., 1997), which is an indicator of bacterial translocation, and LPS bound to AGP is more rapidly cleared from the body than unbound LPS. Consequently, it was surprising to see that neither LMZ nor AAT were associated with AGP, as permeability leading to bacterial translocation has been a principal hypothesized component of EE (Lunn et al., 1991). Other markers of bacterial translocation are needed to confirm these findings.
Although our model indicates that specific enteropathogens alter gut inflammation and permeability, the effect sizes of the pathways from biomarkers to growth were smaller than anticipated (Humphrey, 2009, Brown et al., 2015). Based on available evidence, the contribution of EE to growth deficits as captured in these biomarkers is small relative to the accrued growth deficits in these populations (Lunn et al., 1991, Humphrey, 2009, MAL-ED Network Investigators, n.d, MAL-ED Network Investigators, n.d). The use of additional biomarkers or combinations of biomarkers and growth phenotypes (Naylor et al., 2015) or inclusion of metabolic markers may help to identify stronger support for links between the interactions of enteropathogen pressure and undernutrition on growth failure. Additionally, on-going work in this study population to understand links between the microbiome (Subramanian et al., 2014), host metabolism (Mayneris-Perxachs et al., 2016), and growth will expand our understanding of how microbial populations affect the nutritional status in these populations (Lunn, 2000, Brown et al., 2015, Kau et al., 2015, Blanton et al., 2016, Kosek et al., 2016).
The analytical framework described here is the first attempt to explicitly examine causal pathways of EE. Our results demonstrate that exposure to enteropathogens results in abnormal gut permeability, inflammation, systemic immune activation, and growth failure, but suggest that additional work incorporating other critical features of host metabolic status and the microbiome are needed to explain the gap between the insults attributable to EE and the observed cumulative acquired deficits of growth in children in these populations.
Contributors
Margaret N. Kosek and Benjamin J.J. McCormick devised the model in discussion with Richard L. Guerrant, Laura E. Caulfield, Tahmeed Ahmed, Zulfiquar Bhutta, Gagandeep Kang, Aldo Lima, Eric Houpt, and James Platts-Mills. Benjamin J.J. McCormick ran the analyses. Margaret N. Kosek, Benjamin J.J. McCormick, Gwenyth Lee, and Jessica C. Seidman participated in the interpretation of the results and drafted the manuscript. The MAL-ED Investigators participated in the design, conduct, and analysis of the MAL-ED study and its results.
Declaration of Interests
We declare that we have no conflicts of interest.
Acknowledgements
The Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED) is carried out as a collaborative project supported by the Bill & Melinda Gates Foundation (47075), the Foundation for the National Institutes of Health, and the National Institutes of Health, Fogarty International Center. We particularly want to thank the children and caregivers who participated in the study for their invaluable contributions. Tom Brewer and Gretchen Meller are also thanked for their early support of the project. We are truly indebted to the following individuals for their advisory role in the MAL-ED study: Henry Binder, Maureen Black, Kathleen Braden, Robert Breiman, Susan Bull, Katherine Dewey, Christopher Duggan, Christine Grady, Gerry Keusch, Nancy Krebs, Claudio Lanata, James Nataro, Jerome Singh, Peter Smith, Phillip Tarr, Katherine Tucker and Theodore Wachs. Fraser Lewis is recognized and appreciated for his critical review of and guidance on the analytical methods and statistical approaches applied in the MAL-ED study. We thank Leslie Pray and Alicia Livinski for their editorial assistance. Special thanks go to Roger Glass and George Griffin for their continued support and guidance.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.02.024.
Contributor Information
Margaret N. Kosek, Email: mkosek@jhu.edu.
The MAL-ED Network Investigators:
Tahmeed Ahmed, Zulfiquar Bhutta, Laura Caulfield, Richard Guerrant, Eric Houpt, Gagandeep Kang, Margaret Kosek, Gwenyth Lee, Aldo Lima, Benjamin J.J. McCormick, James Platts-Mills, Jessica Seidman, Laura Caulfield, Margaret Kosek, Gwenyth Lee, Benjamin J.J. McCormick, Jessica Seidman, Rebecca R. Blank, Michael Gottlieb, Stacey L. Knobler, Dennis R. Lang, Mark A. Miller, Karen H. Tountas, Zulfiqar A. Bhutta, Laura Caulfield, William Checkley, Richard L. Guerrant, Eric Houpt, Margaret N. Kosek, Dennis R. Lang, Carl J. Mason, Mark A. Miller, Laura E. Murray-Kolb, William A. Petri, Jr., Jessica C. Seidman, Tahmeed Ahmed, Pascal Bessong, Zulfiqar A. Bhutta, Rashidul Haque, Sushil John, Gagandeep Kang, Margaret N. Kosek, Aldo A.M. Lima, Estomih R. Mduma, Reinaldo B. Oriá, Prakash Sunder Shrestha, Sanjaya Kumar Shrestha, Erling Svensen, Anita K.M. Zaidi, Cláudia B. Abreu, Angel Mendez Acosta, Imran Ahmed, A.M. Shamsir Ahmed, Asad Ali, Ramya Ambikapathi, Leah Barrett, Aubrey Bauck, Eliwaza Bayyo, Ladaporn Bodhidatta, Anuradha Bose, J. Daniel Carreon, Ram Krishna Chandyo, Vivek Charu, Hilda Costa, Rebecca Dillingham, Alessandra Di Moura, Viyada Doan, Jose Quirino Filho, Jhanelle Graham, Christel Hoest, Iqbal Hossain, Munirul Islam, M. Steffi Jennifer, Shiny Kaki, Beena Koshy, Gwenyth Lee, Álvaro M. Leite, Noélia L. Lima, Bruna L.L. Maciel, Mustafa Mahfuz, Cloupas Mahopo, Angelina Maphula, Benjamin J.J. McCormick, Monica McGrath, Archana Mohale, Milena Moraes, Francisco S. Mota, Jayaprakash Muliyil, Regisiana Mvungi, Gaurvika Nayyar, Emanuel Nyathi, Maribel Paredes Olortegui, Reinaldo Oria, Angel Orbe Vasquez, William K. Pan, John Pascal, Crystal L. Patil, Laura Pendergast, Silvia Rengifo Pinedo, James Platts-Mills, Stephanie Psaki, Mohan Venkata Raghava, Karthikeyan Ramanujam, Muneera Rasheed, Zeba A. Rasmussen, Stephanie A. Richard, Anuradha Rose, Reeba Roshan, Barbara Schaefer, Rebecca Scharf, Jessica C. Seidman, Srujan L. Sharma, Binob Shrestha, Rita Shrestha, Suzanne Simons, Alberto M. Soares, Rosa M.S. Mota, Sajid Soofi, Tor Strand, Fahmida Tofail, Rahul J. Thomas, Ali Turab, Manjeswori Ulak, Vivian Wang, Ladislaus Yarrot, Pablo Peñataro Yori, Didar Alam, Ramya Ambikapathi, Caroline Amour, Cesar Banda Chavez, Sudhir Babji, Rosa Rios de Burga, Viyada Doan, Julian Torres Flores, Jean Gratz, Ajila T. George, Dinesh Hariraju, Alexandre Havt, Eric Houpt, Priyadarshani Karunakaran, Robin P. Lazarus, Ila F. Lima, Monica McGrath, Dinesh Mondal, Pedro H.Q.S. Medeiros, Rosemary Nshama, Josiane Quetz, Shahida Qureshi, Sophy Raju, Anup Ramachandran, Rakhi Ramadas, A. Catharine Ross, Mery Siguas Salas, Amidou Samie, Kerry Schulze, Jessica C. Seidman, E. Shanmuga Sundaram, Buliga Mujaga Swema, and Dixner Rengifo Trigoso
Appendix A. Supplementary data
MAL-ED Network Members.
References
- Beltinger J., Del Buono J., Skelly M.M. Disruption of colonic barrier function and induction of mediator release by strains of Campylobacter jejuni that invade epithelial cells. World J. Gastroenterol. 2008;14:7345–7352. doi: 10.3748/wjg.14.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkes J., Viswanathan V.K., Savkovic S.D., Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003;52:439–451. doi: 10.1136/gut.52.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton L.V., Charbonneau M.R., Salih T. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016;351 doi: 10.1126/science.aad3311. 10.1126/science.aad3311 (aad3311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E.M., Wlodarska M., Willing B.P. Diet and specific microbial exposure trigger features of environmental enteropathy in a novel murine model. Nat. Commun. 2015;6:7806. doi: 10.1038/ncomms8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D.I., Lunn P.G., Elia M. Age-related association of small intestinal mucosal enteropathy with nutritional status in rural Gambian children. Br. J. Nutr. 2002;88:499–505. doi: 10.1079/BJN2002697. [DOI] [PubMed] [Google Scholar]
- Campbell D.I., Murch S.H., Elia M. Chronic T cell-mediated enteropathy in rural west African children: relationship with nutritional status and small bowel function. Pediatr. Res. 2003;54:306–311. doi: 10.1203/01.PDR.0000076666.16021.5E. [DOI] [PubMed] [Google Scholar]
- Campbell D.I., McPhail G., Lunn P.G., Elia M., Jeffries D.J. Intestinal inflammation measured by fecal neopterin in Gambian children with enteropathy: association with growth failure, Giardia lamblia, and intestinal permeability. J. Pediatr. Gastroenterol. Nutr. 2004;39:153–157. doi: 10.1097/00005176-200408000-00005. [DOI] [PubMed] [Google Scholar]
- Caulfield L.E., Bose A., Chandyo R.K. Infant feeding practices, dietary adequacy, and micronutrient status measures in the MAL-ED study. Clin. Infect. Dis. 2014;59:S248–S254. doi: 10.1093/cid/ciu421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denno D.M., VanBuskirk K., Nelson Z.C., Musser C.A., Hay Burgess D.C., Tarr P.I. Use of the lactulose to mannitol ratio to evaluate childhood environmental enteric dysfunction: a systematic review. Clin. Infect. Dis. 2014;59:S213–S219. doi: 10.1093/cid/ciu541. [DOI] [PubMed] [Google Scholar]
- George C.M., Oldja L., Biswas S. Geophagy is associated with environmental enteropathy and stunting in children in Rural Bangladesh. Am. J. Trop. Med. Hyg. 2015;92:1117–1124. doi: 10.4269/ajtmh.14-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto R., Mascie-Taylor C.G.N., Lunn P.G. Impact of intestinal permeability, inflammation status and parasitic infections on infant growth faltering in rural Bangladesh. Br. J. Nutr. 2009;101:1509–1516. doi: 10.1017/S0007114508083554. [DOI] [PubMed] [Google Scholar]
- Greenland S., Pearl J., Robins J.M. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- Guerrant R.L., Steiner T.S., Lima A.A.M., Bobak D.A. How intestinal bacteria cause disease. J. Infect. Dis. 1999;179:S331–S337. doi: 10.1086/513845. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Perlot T., Rehman A. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houpt E., Gratz J., Kosek M. Microbiologic methods utilized in the MAL-ED cohort study. Clin. Infect. Dis. 2014;59:S225–S232. doi: 10.1093/cid/ciu413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey J.H. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet. 2009;374:1032–1035. doi: 10.1016/S0140-6736(09)60950-8. [DOI] [PubMed] [Google Scholar]
- Kamada N., Chen G.Y., Inohara N., Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013;14:685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau A.L., Planer J.D., Liu J. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aaa4877. 10.1126/scitranslmed.aaa4877 (276ra24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P., Menzies I., Crane R. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. Am.J.Trop. Med. Hyg. 2004;70:412–419. [PubMed] [Google Scholar]
- Keusch G.T., Rosenberg I.H., Denno D.M. Implications of acquired environmental enteric dysfunction for growth and stunting in infants and children living in low- and middle-income countries. Food Nutr. Bull. 2013;34:357–364. doi: 10.1177/156482651303400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G.T., Denno D.M., Black R.E. Environmental enteric dysfunction: pathogenesis, diagnosis, and clinical consequences. Clin. Infect. Dis. 2014;59:S207–S212. doi: 10.1093/cid/ciu485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpe P.S., Petri W.A., Jr. Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol. Med. 2012;18:328–336. doi: 10.1016/j.molmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosek M., Haque R., Lima A. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am.J.Trop. Med. Hyg. 2013;88:390–396. doi: 10.4269/ajtmh.2012.12-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosek M., Guerrant R.L., Kang G. Assessment of environmental enteropathy in the MAL-ED cohort study: theoretical and analytic framework. Clin. Infect. Dis. 2014;59:S239–S247. doi: 10.1093/cid/ciu457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosek M.N., Mduma E., Kosek P.S. Plasma Tryptophan and the Kynurenine-Tryptophan Ratio are Associated with the Acquisition of Statural Growth Deficits and Oral Vaccine Underperformance in Populations with Environmental Enteropathy. Am. J. Trop. Med. Hyg. 2016 doi: 10.4269/ajtmh.16-0037. 10.4269/ajtmh.16-0037 (published online Aug 8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer J.M., Wilting J., Janssen L.H. Drug binding to human alpha-1-acid glycoprotein in health and disease. Pharmacol. Rev. 1988;40:1–47. [PubMed] [Google Scholar]
- Lin A., Arnold B.F., Afreen S. Household environmental conditions are associated with enteropathy and impaired growth in rural Bangladesh. Am.J.Trop. Med. Hyg. 2013;89:130–137. doi: 10.4269/ajtmh.12-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann T.G., Roche A.F., Martorell R. Human Kinetics Books; Champaign, IL: 1988. Anthropometric Standardization Reference Manual. [Google Scholar]
- Lunn P.G. The impact of infection and nutrition on gut function and growth in childhood. Proc. Nutr. Soc. 2000;59:147–154. doi: 10.1017/s0029665100000173. [DOI] [PubMed] [Google Scholar]
- Lunn P.G., Northrop-Clewes C.A., Downes R.M. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet. 1991;338:907–910. doi: 10.1016/0140-6736(91)91772-m. [DOI] [PubMed] [Google Scholar]
- MAL-ED Network Investigators The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clin. Infect. Dis. 2014;59:S193–S206. doi: 10.1093/cid/ciu653. [DOI] [PubMed] [Google Scholar]
- MAL-ED Network Investigators The Malnutrition and Enteric Disease Study (MAL-ED): understanding the consequences for child health and development. Clin. Infect. Dis. 2014;59:S193–S330. [Google Scholar]
- MAL-ED Network Investigators. Growth velocity and attained size at 24 months are affected by enteropathogens and dietary intakes, but not diarrhoea: findings from the MAL-ED birth cohort study. Lancet (submitted).
- MAL-ED Network Investigators. Prevalence of stunting at 24 months is linked to enteropathogens, diet, socioeconomic status, maternal height, and child weight at enrolment: findings from the MAL-ED birth cohort study. Lancet (submitted).
- MAL-ED Network Investigators. Oral polio vaccine failure is associated with socio-environmental conditions, enteropathogens, and timing and frequency of vaccination: findings from the MAL-ED birth cohort study. Personal Communication.
- Mayneris-Perxachs J., Lima A.A.M., Guerrant R.L. Urinary N-methylnicotinamide and β-aminoisobutyric acid predict catch-up growth in undernourished Brazilian children. Sci. Rep. 2016;6:19780. doi: 10.1038/srep19780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick B.J.J., Lee G.O., Seidman J.C. Dynamics and trends in fecal biomarkers of gut function in children from 1–24 months in the MAL-ED study. Am.J.Trop. Med. Hyg. 2016 doi: 10.4269/ajtmh.16-0496. (16–0496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies I.S., Zuckerman M.J., Nukajam W.S. Geography of intestinal permeability and absorption. Gut. 1999;44:483–489. doi: 10.1136/gut.44.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers S., Wolke A., Field S.P., Feuer E.J., Johnson J.W., Janowitz H.D. Fecal alpha 1-antitrypsin measurement: an indicator of Crohn's disease activity. Gastroenterology. 1985;89:13–18. doi: 10.1016/0016-5085(85)90739-5. [DOI] [PubMed] [Google Scholar]
- Mondal D., Minak J., Alam M. Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin. Infect. Dis. 2012;54:185–192. doi: 10.1093/cid/cir807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D.F., Rosenfeld M.R., Gribbon P.M., Winlove C.P., Tsai C.M. Alpha-1-acid (AAG, orosomucoid) glycoprotein: interaction with bacterial lipopolysaccharide and protection from sepsis. Inflammation. 1997;21:69–82. doi: 10.1023/a:1027342909423. [DOI] [PubMed] [Google Scholar]
- Naylor C., Lu M., Haque R. Environmental enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine. 2015;2:1759–1766. doi: 10.1016/j.ebiom.2015.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl J. Causal diagrams for empirical research. Biometrika. 1995;82:669–688. [Google Scholar]
- Peterson K.M., Buss J., Easley R. REG1B as a predictor of childhood stunting in Bangladesh and Peru. Am. J. Clin. Nutr. 2013;97:1129–1133. doi: 10.3945/ajcn.112.048306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platts-Mills J.A., Babji S., Bodhidatta L. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED) Lancet Glob. Health. 2015 doi: 10.1016/S2214-109X(15)00151-5. 10.1016/S2214-109X(15)00151-5 (published online July 17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer M. Proceedings of the 3rd International Workshop on Distributed Statistical Computing (DSC 2003) March 2003. JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling; pp. 20–22. [Google Scholar]
- Prendergast A., Kelly P. Enteropathies in the developing world: neglected effects on global health. Am.J.Trop. Med. Hyg. 2012;86:756–763. doi: 10.4269/ajtmh.2012.11-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard S.A., Barrett L.J., Guerrant R.L., Checkley W., Miller M.A. Disease surveillance methods used in the 8-site MAL-ED cohort study. Clin. Infect. Dis. 2014;59:S220–S224. doi: 10.1093/cid/ciu435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki T. Myeloperoxidase concentrations in the stool as a new parameter of inflammatory bowel disease. Kurume Med. J. 1998;45:69–73. doi: 10.2739/kurumemedj.45.69. [DOI] [PubMed] [Google Scholar]
- Semba R.D., Shardell M., Sakr Ashour F.A. Child stunting is associated with low circulating essential amino acids. EBioMedicine. 2016;6:246–252. doi: 10.1016/j.ebiom.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S., Huq S., Yatsunenko T. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510:417–421. doi: 10.1038/nature13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan V.K., Hodges K., Hecht G. Enteric infection meets intestinal function: how bacterial pathogens cause diarrhoea. Nat. Rev. Microbiol. 2009;7:110–119. doi: 10.1038/nrmicro2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz A.J., Manary M.J., Stephenson K. Abnormal gut integrity is associated with reduced linear growth in rural Malawian children. J. Pediatr. Gastroenterol. Nutr. 2012;55:747–750. doi: 10.1097/MPG.0b013e3182650a4d. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva, Switzerland: 2006. WHO Child Growth Standards: Methods and development: Length/height-for-age, Weight-for-age, Weight-for-length, Weight-for-height and Body Mass Index-for-age. ( http://www.who.int/childgrowth/publications/technical_report_pub/en/ accessed July 23, 2013) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MAL-ED Network Members.