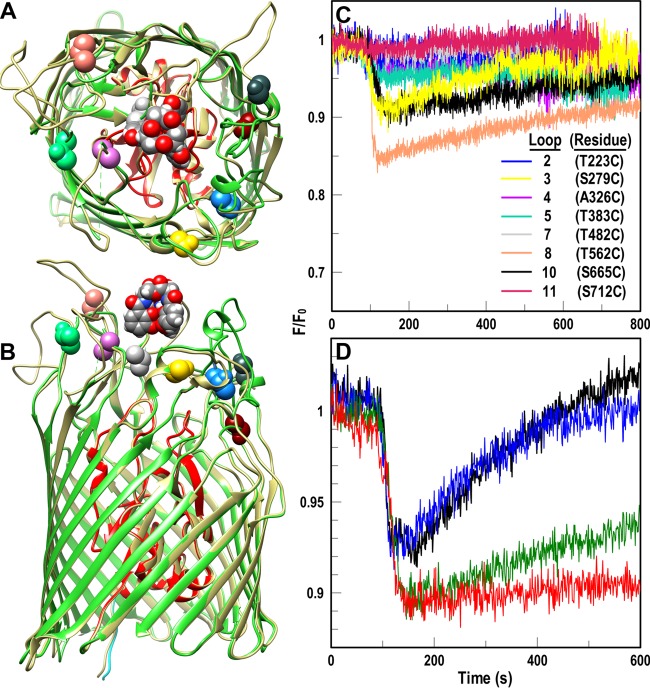
FIG 5.
Fluoresceination of Cys substitutions in AbaFepA facilitates measurement of FeEnt uptake by A. baumannii. (A and B) Cys mutagenesis of AbaFepA. The Modeller function of CHIMERA (48) predicted the tertiary structure of AbaFepA (dark beige) based on its 46% identity to EcoFepA (PDB file 1FEP; N domain, red; C domain, lime green). We selected eight amino acids in the surface loops of AbaFepA loops (colored and depicted in space-filling format) with Cys, from their similarity to residues in EcoFepA that when fluoresceinated gave substantial quenching during FeEnt binding (28). (C and D) Fluorescence spectroscopic measurement of FeEnt transport in A. baumannii. (C) After transforming mutant plasmids encoding AbaFepA Cys mutants into A. baumannii 17978 ΔfepA, we maximized expression of the AbaFepA Cys mutants by growth in iron-deficient MOPS medium and labeled the cells with FM. We monitored fluorescence voltage from FM-labeled ΔfepA/pAbafepA-Cys mutants in response to addition of 10 nM FeEnt at 100 s and normalized the data to the initial fluorescence of the bacteria (F/F0). Binding of FeEnt to AbaFepAS279C-FM, T562C-FM and S665C-FM quenched their fluorescence; subsequent FeEnt uptake by the cells depleted the ferric siderophore from solution, resulting in a gradual increase to initial fluorescence levels (recovery). The plotted data are the averages from three separate experiments. (D) Normalized fluorescence of FM-labeled 17978 ΔfepA/pAbafepAS279C following exposure to various concentrations of CCCP (black, no CCCP; blue, 2.5 μM CCCP; green, 5 μM CCCP; red, 10 μM CCCP). The plotted data are the averages from two separate experiments.