FIG 8 .
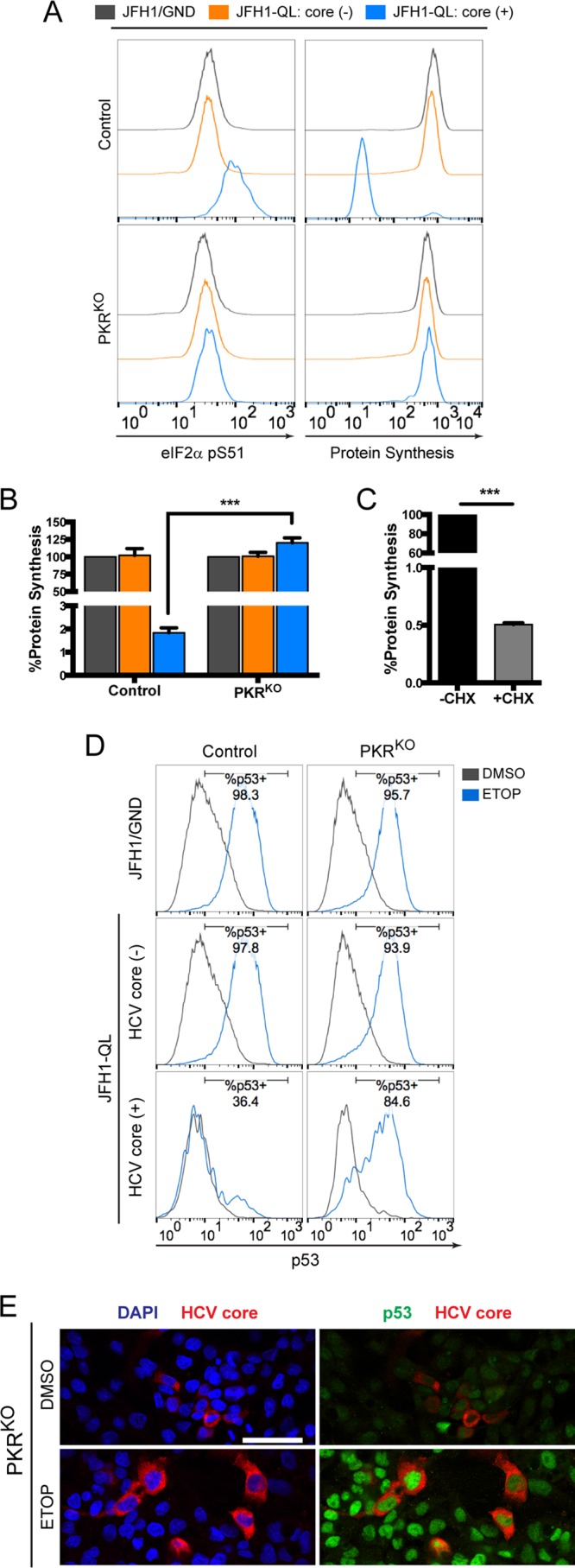
PKR is required for HCV-mediated inhibition of p53. (A) Levels of phosphorylated eIF2α (pS51) (left) and global protein synthesis (right) in HCV core (-) and HCV core (+) populations of control versus PKRKO cells. (B) MFI values for global protein synthesis from the indicated populations of control and PKRKO cell lines are expressed as the percentages of protein synthesis relative to JFH1/GND-electroporated controls. (C) Control cells were labeled in the presence or absence of the translational inhibitor cycloheximide (CHX) (50 μg/ml) to confirm specificity for newly synthesized proteins. Values in panels B and C represent the means ± SEM from three independent experiments. ***, P < 0.0001 by two-way ANOVA with Bonferroni’s correction for multiple comparisons. (D) p53 accumulation in HCV core (-) and HCV core (+) populations of control versus PKRKO cells electroporated with the indicated HCV RNA and treated 72 h later with 100 μM etoposide (ETOP) or DMSO for 2 h. Numbers indicate the percentages of p53-positive cells following etoposide treatment. (E) Immunofluorescence confocal microscopy for p53 and HCV core protein in JFH1-QL RNA-electroporated PKRKO cells treated as described for panel D. Nuclei were labeled with DAPI. Bar, 50 μm.
