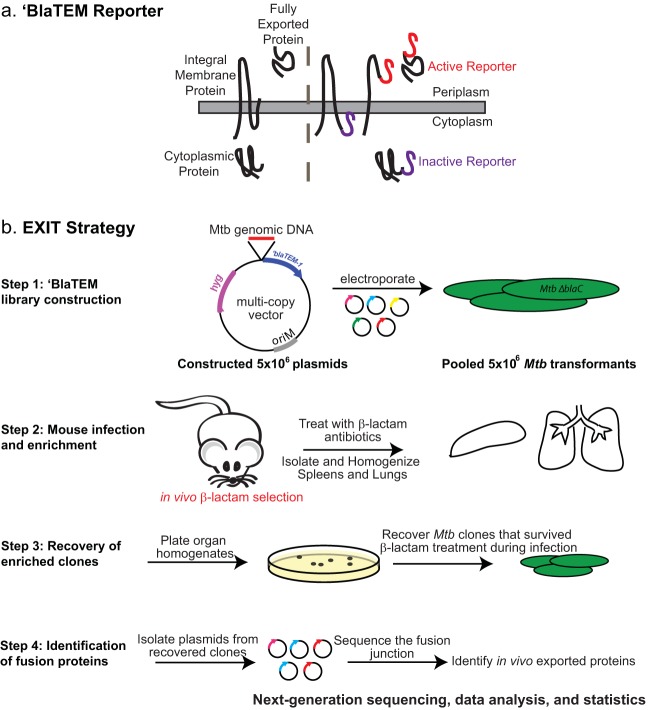
FIG 1 .
(a) The ‘BlaTEM reporter. The ‘BlaTEM reporter is compatible with proteins localized to the bacterial cytoplasmic membrane or cell wall or secreted from the bacterial cell. The right panel indicates in-frame fusions to categories of exported proteins that confer β-lactam resistance (red). In-frame fusions to cytoplasmic proteins or the cytoplasmic domain of integral membrane proteins (purple) do not confer β-lactam resistance. (b) EXIT strategy. In step 1, a comprehensive library of 5 × 106 plasmids containing fragments of M. tuberculosis (Mtb) genomic DNA fused to the ‘blaTEM reporter was constructed. The plasmid library was transformed into the ΔblaC β-lactamase-sensitive mutant of M. tuberculosis, and 5 × 106 transformants were pooled to generate the EXIT library. In step 2, mice were infected by intravenous injection with the EXIT library and treated with β-lactam antibiotics (oral gavage twice daily) to select for EXIT clones exporting ‘BlaTEM fusion proteins. β-lactam treatment began 1 day after infection and continued to 2 weeks after infection. Mice were sacrificed, and spleens and lungs were harvested and homogenized. In step 3, organ homogenates were plated on 7H10 agar and grown to recover M. tuberculosis clones that survived β-lactam treatment during infection. Plates were scraped, and colonies were pooled separately for lungs and spleens. In step 4, plasmids from the recovered bacteria and the input samples were isolated and the fusion junction was sequenced using next-generation sequencing. Sequencing primers were designed to read out of the ‘blaTEM reporter and sequence the immediately adjacent M. tuberculosis DNA. Sequences were aligned to the M. tuberculosis genome. Unique sequences were counted to identify the abundance of each fusion junction site within the population. The genes that were most highly abundant after in vivo β-lactam treatment were identified, and the results corresponded to plasmids producing in-frame exported ‘BlaTEM fusion proteins.