FIG 3 .
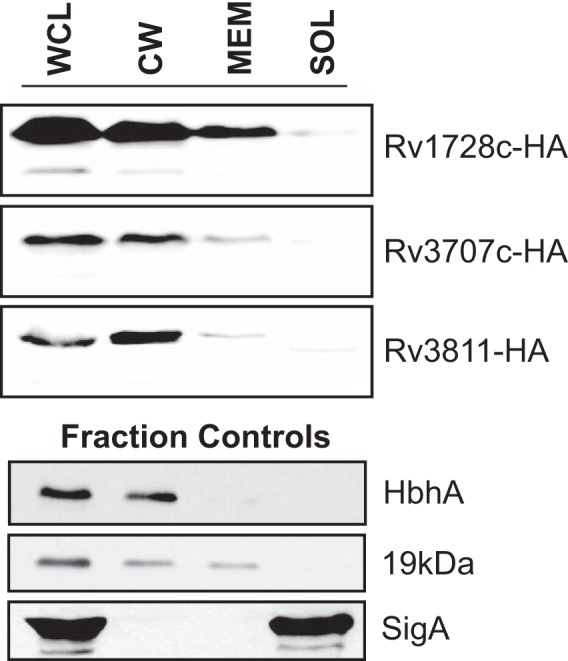
Validation of EXIT-identified exported proteins with no in silico predicted export signal. Three proteins with no in silico predicted export signal (Rv1728c, Rv3707c, and Rv3811) were engineered with C-terminal HA tags and expressed from the constitutive hsp60 promoter in M. tuberculosis. Cells were irradiated, lysed by the use of a French pressure cell into whole-cell lysate (WCL), equalized by bicinchoninic acid (BCA) protein quantification, and fractionated by differential ultracentrifugation into cell wall (CW), membrane (MEM), and soluble/cytoplasmic (SOL) fractions. Fractions derived from equivalent amounts of starting cellular material were separated by SDS-PAGE, and HA-tagged proteins were detected by immunoblotting performed with anti-HA antibodies. The cell wall protein (HbhA), membrane protein (19-kDa lipoprotein), and cytoplasmic protein (SigA) were included as fractionation controls.
