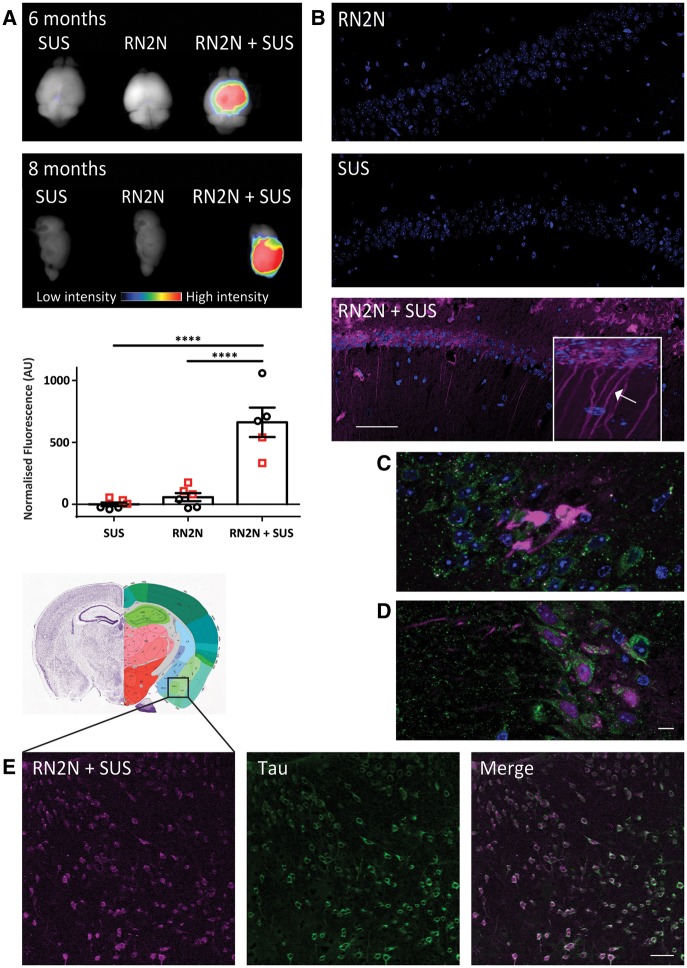
Figure 4.
SUS enhances RN2N scFv delivery across the blood–brain barrier and into neurons. pR5 mice under anaesthesia were injected retro-orbitally with either microbubbles only (SUS), RN2N conjugated to Alexa 647 only (RN2N) or a combination of both (RN2N + SUS). SUS was then applied to SUS and RN2N + SUS groups. After 30 min mice were sacrificed and tissue analysed by fluorescence imaging. (A) Fluorescence imaging indicates widespread brain uptake of RN2N in 6-month and 8-month-old RN2N + SUS treated mice. Quantification of the fluorescent intensity demonstrates an approximate 11-fold increase in scFv uptake in the RN2N + SUS treated mice (mean = 662 ± 119) compared to the RN2N only treated mice (mean = 58 ± 32) (grouping 6-month-old mice = red squares; and 8-month-old mice = black circles; mean ± SEM, P < 0.0001, one-way ANOVA with Tukey’s post-test). (B) Post-sectioning, RN2N was only detectable in the RN2N + SUS treated mice as shown for the CA1 region of the hippocampus. Inset: Zoomed-in image of RN2N + SUS treated mouse brain tissue with RN2N observed within neuronal cell bodies and apical dendrites (arrow). (C) Co-immunofluorescence staining for EEA1 (green) and RN2N (magenta) and (D) LAMP1 (green) and RN2N (magenta) indicates that the internalized RN2N is not confined to endosomes or lysosomes (Scale bar = 10 μm). (E) In the RN2N + SUS treated mice, RN2N is also observed in the amygdala where it co-localized with HT7 staining (human tau). Coronal image sourced from Allen Mouse Brain Atlas (2004) (Scale bar = 50 μm).