Table 1.
Overview of enrolment, interventions, and assessments
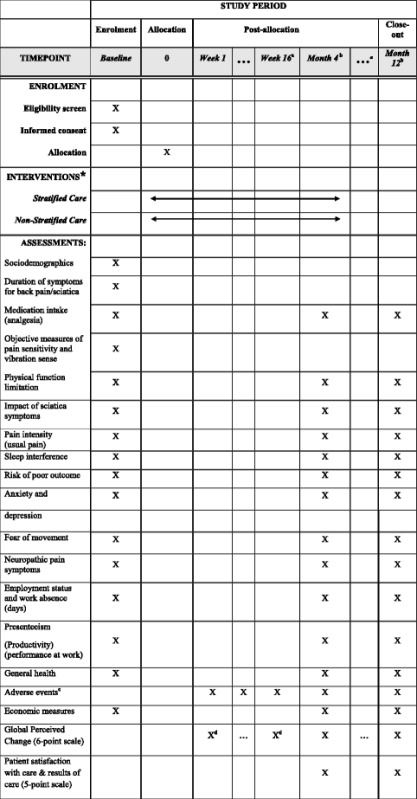
*The interventions and their delivery are described in detail in the manuscript. Treatments are tailored to the individual participant and likely to have different frequency and duration for each participant. Overall, treatment for most participant is expected to be completed within 4 months from randomization
a The trial’s primary outcome is time to symptoms resolution. Data collection with text messages for the primary outcome occurs weekly for the first 4 months for all participants. Between 4 and 12-month follow-up, the text message data collection changes to once every 4 weeks, or until ‘stable resolution’ of symptoms, which is defined as 2 consecutive months’ responses of ‘completely recovered’ or ‘much better’. After stable resolution, data collection for the primary outcome via text message ceases
b At 4 and 12 months follow-up, data collection is via postal questionnaires
c Participants and clinicians are asked to report any adverse and/or serious adverse events. Patients are asked about adverse events in the follow-up questionnaires
d Global perceived change is collected via text messages or telephone calls, and in the follow-up questionnaires at 4 and 12 months
