Abstract
Cognitive disorders of aging represent a serious threat to the social and economic welfare of current society. It is now widely recognized that pathology related to such conditions, particularly Alzheimer’s disease, likely begins years or decades prior to the onset of clinical dementia symptoms. This revelation has led researchers to consider candidate mechanisms precipitating the cascade of neuropathological events that eventually lead to clinical Alzheimer’s disease. Insulin, a hormone with potent effects in the brain, has recently received a great deal of attention for its potential beneficial and protective role in cognitive function. Insulin resistance, which refers to the reduced sensitivity of target tissues to the favorable effects of insulin, is related to multiple chronic conditions known to impact cognition and increase dementia risk. With insulin resistance-associated conditions reaching epidemic proportions, the prevalence of Alzheimer’s disease and other cognitive disorders will continue to rise exponentially. Fortunately, these chronic insulin-related conditions are amenable to pharmacological intervention. As a result, novel therapeutic strategies that focus on increasing insulin sensitivity in the brain may be an important target for protecting or treating cognitive decline. The following review will highlight our current understanding of the role of insulin in brain, potential mechanisms underlying the link between insulin resistance and dementia, and current experimental therapeutic strategies aimed at improving cognitive function via modifying the brain’s insulin sensitivity.
Keywords: Alzheimer’s disease, insulin, cognition, diabetes, aging
1. Introduction
Insulin, long considered to be a hormone that primarily exerts its influence in the periphery, is now known to have potent effects in the brain. Insulin resistance refers to the reduced ability of insulin to exert its action on target tissues and is associated with neuropathological processes that underlie cognitive aging and dementia. Several risk factors implicated in cognitive decline, including diabetes, hyperlipidemia, hypertension, and obesity, are closely linked to underlying insulin dysfunction. Given the rise in such chronic conditions, in combination with a rapidly aging population, the prevalence of Alzheimer’s disease and other cognitive disorders is likely to continue to rise exponentially. Recent clinical trials have focused on identification of novel potential therapeutic mechanisms aimed at improving insulin sensitivity in the brain; with the expectation that these mechanisms may represent promising treatments for cognitive disorders of aging. This review will focus on the effects of insulin in the central nervous system, the relationship between insulin-associated conditions and dementia, and potential mechanisms for the pharmacological treatment of insulin resistance in brain.
2. The role of insulin in the brain
Insulin, a peptide secreted by pancreatic beta cells, is readily transported into the central nervous system across the blood brain barrier by a saturable, receptor-mediated process (Banks et al., 1997a; Baskin et al., 1987; Baura et al., 1993). Insulin receptors are abundant in the synapses of both astrocytes and neurons, and are selectively distributed in the olfactory bulb, cerebral cortex, hippocampus, hypothalamus, amygdala, and septum (Baskin et al., 1987; Havrankova et al., 1978a; Havrankova et al., 1978b; Unger et al., 1991). Here, insulin signaling contributes to both synaptogenesis and synaptic remodeling (Abbott et al., 1999).
Beyond direct effects at the receptor site, insulin exerts additional neuromodulatory actions that have implications for cognition. For example, insulin may augment the induction of long-term potentiation, thought to be the primary cellular basis for memory formation, by influencing cell membrane expression of N-methyl-D-aspartate receptors (Skeberdis et al., 2001). Insulin also regulates expression of the neurotransmitters acetylcholine and norepinephrine, both of which are known to influence cognition (Figlewicz et al., 1993; Kopf and Baratti, 1999). Further, insulin may act to increase cortical cerebral glucose metabolism in brain regions important for learning and memory (Bingham et al., 2002). Regional insulin effects on glucose metabolism may be related to the distribution of insulin-sensitive glucose transporter isoforms 4 and 8 (Reagan et al., 2001; Schulingkamp et al., 2000), which in the rat brain are selectively distributed in the cerebellum, sensorimotor cortex, hippocampus, pituitary, and hypothalamus (glucose transporter isoform 4) and in the hippocampus and hypothalamus (glucose transporter isoform 8) (Reagan et al., 2001). Insulin has been shown to increase brain glucose transporter isoform 4 expression and translocation (Piroli et al., 2007), and substantial co-localization exists for insulin-containing neurons, insulin receptors and both glucose transporter isoforms 4 and 8 (Apelt et al., 1999; Schulingkamp et al., 2000).
Given numerous influences of insulin in selective brain regions, including medial temporal lobe structures that support learning and memory, it is not surprising that there is a growing literature that links insulin to cognitive function. In both rat models and human studies, acute insulin administration reliably improves memory (Unger et al., 1991) (Craft et al., 2003; Craft et al., 1999; Craft et al., 1996; Kern et al., 2001). Interestingly, the learning process itself is also accompanied by changes in insulin signaling molecules in the hippocampus. This was demonstrated by increased insulin receptor expression in the dentate gyrus and hippocampal CA1 field in rodents trained on a spatial memory task (Zhao et al., 1999). Together, these studies support that insulin may play an important role in regulating normal cognitive function.
3. Insulin resistance and Alzheimer’s disease pathology
While acute hyperinsulinemia may produce beneficial effects on cognition, persistently high levels of circulating insulin may conversely exert a negative influence on memory and other cognitive functions. Raising peripheral insulin levels acutely elevates brain and cerebrospinal fluid insulin levels, whereas prolonged peripheral hyperinsulinemia down-regulates insulin receptors at the blood-brain barrier and reduces insulin transport into the brain (Schwartz et al., 1990; Wallum et al., 1987). Chronically elevated insulin can thus lead to insulin resistance, characterized by high peripheral insulin and diminished insulin-mediated glucose clearance (Reaven, 1983, 2003). Such high levels of insulin over time can render tissue unresponsive to insulin, resulting in substantial damage to muscle, liver, adipose tissue, endothelium, and brain. The insulin resistance syndrome has been linked to several chronic medical conditions, including cardiovascular disease, metabolic syndrome, diabetes, and increasingly, dementia. However, the pancreas is able to compensate by generating adequate levels of insulin to maintain lower glucose levels for some time prior to the onset of frank disease. Thus, abnormally high levels of peripheral insulin may be silently contributing to the initiation of the Alzheimer’s disease pathology cascade in some individuals years or decades prior to initial clinical dementia symptoms (Xu et al., 2009).
3.1. Evidence for insulin resistance in the Alzheimer brain
Hoyer and colleagues were the first to suggest that desensitization of the neuronal insulin receptor plays a role in Alzheimer’s disease by demonstrating a reduction in insulin receptors and tyrosine kinase activity markers in the Alzheimer brain. (Frolich et al., 1998; Hoyer, 2002) Subsequently, reduced insulin and insulin-like growth factor 1 message was shown to correlate with increasing Alzheimer pathology and cholinergic deficit (Rivera et al., 2005). Recently, through a series of ex vivo experiments in autopsied cognitively normal participants and patients with Alzheimer’s disease, brain insulin resistance was demonstrated (particularly in the hippocampus) and was associated with clinical symptoms of cognitive decline prior to death (Talbot et al., 2012). Such insulin resistance was determined to be independent of type 2 diabetes diagnosis or apolipoprotein E genotype. Collectively, these findings provide support that Alzheimer’s disease is associated with insulin resistance in the brain.
3.2. Insulin regulation of beta amyloid
Increasingly, in vitro and animal studies show that insulin has robust effects at multiple points during the amyloid cascade, and thus in all likelihood can modulate the process by which the beta amyloid (Aβ) peptide aggregates in the brain. In both rat and human cell cultures, insulin reduces the phosphorylation of amyloid precursor protein and increases proteins that are anti-amyloidogenic in nature, including insulin degrading enzyme, β-secretase, and glycogen synthase kinase-3β (Pandini et al., 2013). Further, insulin fosters the release of intracellular Aβ in neuronal cultures, affecting both its short (Aβ40) and long (Aβ42) forms and accelerating their trafficking from the Golgi and trans-Golgi network to the plasma membrane (Gasparini et al., 2001; Pandini et al., 2013). Thus, in the presence of reduced brain insulin, the trafficking of Aβ from intracellular to extracellular compartments may be compromised.
Increasingly, the role of impaired Aβ clearance, rather than increased production of Aβ, has been recognized as a key factor in the development of late-onset Alzheimer’s neuropathology. Insulin degrading enzyme, a metalloprotease that catabolizes insulin, is highly expressed in brain (Authier et al., 1996) and is recognized as having an essential role in both Aβ clearance (Kurochkin and Goto, 1994; McDermott and Gibson, 1997; Qiu et al., 1998; Zhao et al., 2004) and intracellular degradation (Sudoh et al., 2002). Postmortem brain tissue from Alzheimer’s patients shows lower overall levels of insulin degrading enzyme and messenger ribonucleic acid, as well as reduced insulin degrading enzyme activity. In addition, insulin degrading enzyme knockout mice have reduced insulin in brain as well as muted degradation of Aβ and insulin in brain (Cook et al., 2003; Farris et al., 2003; Perez et al., 2000). Thus, through reducing levels of insulin degrading enzyme in brain, low levels of insulin in the central nervous system may interfere with Aβ clearance.
Chronic peripheral hyperinsulinemia has been associated with a pattern in which brain insulin levels are initially higher, then decrease as transport of insulin into the brain is down-regulated (Banks et al., 1997b). Consistent with this pattern, it has been shown that genetically obese Zucker rats have reduced insulin binding to brain capillaries (Schwartz et al., 1990) and reduced hypothalamic insulin levels (Gerozissis et al., 1993) in comparison with lean controls. Additionally, brain uptake of labeled insulin was reduced and peripheral insulin clearance was inhibited in insulin resistant canines (Kaiyala et al., 2000). Adults with Alzheimer’s disease show lower insulin levels in cerebrospinal fluid, higher plasma insulin levels, and reduced cerebrospinal fluid to plasma insulin ratios compared to healthy controls (Craft et al., 1998). High plasma insulin levels may interfere with degradation of Aβ transported out of the brain, thereby obstructing a peripheral Aβ-clearing “sink.” Concomitantly, low brain insulin levels reduce release of Aβ from intracellular compartments into extracellular compartments where clearance is believed to occur. Thus, for some patients with Alzheimer’s disease, high peripheral insulin levels and low brain insulin levels would result in reduced clearance of Aβ both in brain and in the periphery.
Support for the validity of this model is provided by a recent study that induced insulin resistance in the transgenic 2576 (Tg2576) mouse model of Alzheimer’s disease with a high fat diet. Diet manipulation resulted in a metabolic profile of high peripheral insulin, and low brain insulin and insulin degrading enzyme levels compared with Tg2576 mice fed a normal diet (Ho et al., 2004). Diet-induced insulin resistance caused two-fold increases in Aβ40 and 42, and earlier, larger Aβ deposits compared with non-insulin resistant Tg2576 mice. Furthermore, insulin resistant mice had impaired learning on a water maze test. These results are consistent with a subsequent study showing that insulin regulates insulin degrading enzyme and Aβ through a phosphatidylinositol-3-kinase-dependent signaling mechanism (Zhao et al., 2004), in which treatment of primary hippocampal neurons with insulin produced a 25 percent increase in insulin degrading enzyme expression. In another model of insulin resistance, amyloid precursor protein/presenilin-1 transgenic mice were given sucrose sweetened beverages, and also demonstrated increased brain Aβ deposition and reduce Morris water maze learning (Cao et al., 2007). Together these results suggest that insulin resistance can precipitate the neuropathological and behavioral features of Alzheimer’s disease, and that raising brain insulin levels may reduce neuropathological changes related to the disease.
3.3. Aβ regulation of brain insulin signaling
Conversely, Aβ also regulates insulin signaling in the brain. Soluble Aβ binds to the insulin receptor and disrupts its signaling capacity and induction of long-term potentiation in mouse hippocampal slice preparations (Townsend et al., 2007). These effects were prevented by pre-treatment of the tissue with insulin prior to Aβ exposure. Synthetic soluble Aβ oligomers, known as Aβ-derived diffusible ligands, downregulate plasma membrane insulin receptors in primary hippocampal cultured neurons, leading to synaptic spine loss. This process was also prevented by pre-treatment with insulin (De Felice et al., 2009). A related mechanism through which insulin and Aβ may interact to modulate Alzheimer’s pathology is via synaptotoxic effects. Loss of synapses is the earliest structural defect observed in Alzheimer’s disease. Soluble oligomeric species of Aβ are synaptotoxic, and insulin prevents binding of Aβ to synapses, thereby preserving synaptic integrity (De Felice et al., 2009). Insulin also reduces oligomer formation which may have additional protective effects; a functional consequence of these effects appears to be protection against Aβ-induced disruption of long term potentiation integrity, the process of synaptic remodeling believed to underlie memory formation (Lee et al., 2009). Collectively, these findings suggest that soluble Aβ may induce neuronal insulin resistance and synapse loss.
3.4. Other Alzheimer’s-related mechanisms
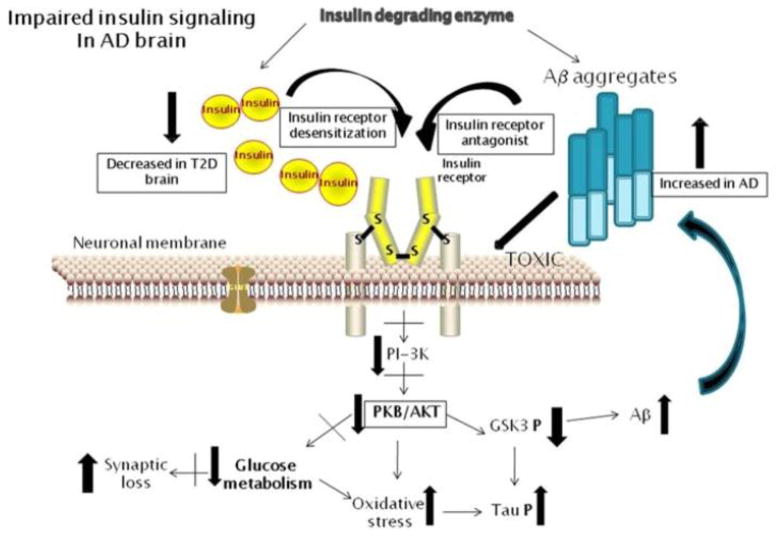
In addition to insulin’s role in Aβ clearance and degradation, insulin also inhibits phosphorylation of tau, the protein the forms neurofibrillary tangles, a neuropathological hallmark of Alzheimer’s disease and other dementing processes. Insulin may affect tau through its regulation of glycogen synthase kinase 3β, a downstream target in the insulin signaling pathway (Hong and Lee, 1997). Schubert and colleagues (Schubert et al., 2004) abolished insulin signaling in vivo with a conditional knockout mouse model in which the insulin receptor gene was inactivated in the central nervous system. Phosphorylation of glycogen synthase kinase 3β and Akt was reduced and phosphorylation of tau increased 3.5-fold. Recent work also implicates insulin receptor substrate 2, in that mice in which this gene has been knocked out have increased tangles and hyperphosphorylated tau (Schubert et al., 2003). Further, insulin dysregulation is also associated with oxidative stress, inflammation, and impaired neurogenesis (Craft and Watson, 2004). Figure 1 demonstrates the potential consequences of insulin resistance on Alzheimer pathology in the brain.
Figure 1.
Consequences of insulin and Aβ interactions on reduced neuronal insulin receptor signaling and promoting Alzheimer’s disease pathology. In type 2 diabetes, there can be decreased or increased levels of insulin in brain (depending on disease state), along with insulin receptor desensitization. Aβ peptide levels can be enhanced by reduced insulin receptor signaling, and soluble Aβ oligomers can also block these receptors. Increased Aβ levels will compete for insulin degrading enzyme with cerebral insulin. Aβ aggregates can also have direct membrane toxic effect on neuronal cells. Reduced insulin receptor signaling results in reduced phosphoinositide-3 kinase activity that leads to reduced protein kinase B/Akt activity. The consequences of this include reduced glucose metabolism and increased oxidative stress. Specifically reduced glycogen synthase kinase-3 phosphorylation leads to increased tau phosphorylation and Aβ formation.
4. Insulin resistance-related conditions and dementia
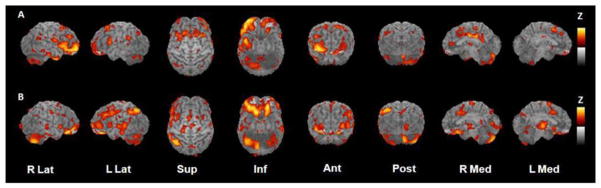
Above, we provide compelling evidence that insulin resistance alone is a viable candidate risk factor for Alzheimer’s disease. Indeed, recent studies have associated insulin resistance with Alzheimer-consistent impairments on functional imaging scans. For example, insulin resistance (defined as having high levels of fasting plasma insulin) in middle-aged women was associated with reduced activity in the prefrontal cortex and hippocampal regions, which corresponded with reductions in performance on neuropsychological test performance (Kenna et al., 2013). Reductions in cerebral glucose metabolism in prefrontal, temporal, and cingulate regions identified by fludeoxyglucose F 18-positron emission tomography were noted in prediabetic and diabetic older adults as compared to nondiabetic adults (Baker et al., 2011) (Figure 2). Despite these small experimental studies, because insulin resistance is often not identified in its earliest stages in the absence of its related chronic conditions, it is often not studied in population-based models. Thus, in the following sections, we focus on the increased dementia risk associated with insulin resistance-related syndromes.
Figure 2.
Statistical parametric maps of task-specific activation for (A) normal adults and (B) adults with prediabetes/type 2 diabetes. Maps were constructed by subtracting resting scans from scans obtained while participants performed a memory encoding task. Yellow represents areas of greatest activation.
4.1. Diabetes
The repeated connection between insulin and mechanisms related to Alzheimer’s disease pathology have led some to refer to Alzheimer’s disease as “Type 3 diabetes” (de la Monte and Wands, 2008). Indeed, Type 2 diabetes predicts cognitive decline in older adults (Tilvis et al., 2004) and confers a significantly increased risk of both Alzheimer’s disease and vascular dementia (Luchsinger, 2008; Strachan et al., 2008). A recent meta-analysis found that 14 of 15 community-based studies reported positive associations between diabetes and Alzheimer’s disease, with a pooled population-attributable risk of 8% (Tolppanen et al., 2013). In the prospective, community-based Rotterdam study, Ott et al.(Ott et al., 1996) found that Type 2 diabetes significantly increased the risk for all-cause dementia and Alzheimer’s disease, with greater risk apparent in people who were insulin-treated (and therefore likely to be in the more severe stages of the disease) at baseline. Similar results were reported by Leibson et al (Leibson et al., 1997) and the Religious Orders Study reported a 65% increased risk for Alzheimer’s disease among those with Type 2 diabetes (Arvanitakis et al., 2004). Findings from the Mayo Clinic Alzheimer’s Disease Patient Registry show an increased prevalence of diabetes (35% vs. 18% in non-demented control subjects) and impaired glucose tolerance (46% vs. 24%) for patients with Alzheimer’s disease (Janson et al., 2004). Further, Alzheimer’s risk is raised independently from vascular or other dementias (Maher and Schubert, 2009; Ott et al., 1999), a finding that is not surprising given the wealth of literature that connects insulin dysfunction with Alzheimer-specific brain pathology.
Despite the associations between cognitive decline and type 2 diabetes, recent neuropathological studies have produced somewhat conflicting results. One study showed that dementia patients with treated diabetes had Aβ plaque loads that were similar to those of non-demented controls, while untreated diabetic dementia patients had plaque loads consistent with dementia patients without diabetes (Beeri et al., 2008). Treated diabetics with dementia had higher levels of microvascular infarcts and antiinflammatory markers to a degree not present in untreated diabetics (Sonnen et al., 2009). Given the preliminary nature of these results and small sample sizes, these studies must be replicated prior to making any firm conclusions as to their meaning. If supported by larger studies, however, these findings could bring into question the relative impact of both Aβ and microvascular disease in the development of clinical dementia symptoms. It is possible that treated diabetics, who are likely to be at a more advanced stage of disease, express the symptoms of dementia at lower levels of amyloid burden due to co-existing microvascular damage. Future neuropathological studies that carefully examine disease duration, treatment duration and dose, and concomitant vascular risk factors will certainly help to clarify these questions.
4.2 Dyslipidemia
Insulin resistance is associated with dyslipidemia, and lipid metabolism may be related to Aβ accumulation. Animal models show greater very low density lipid secretion in advance of Aβ deposits in the brain (Burgess et al., 2006), and Alzheimer’s disease is associated with increased post-prandial chylomicron and low density lipid levels (Mamo et al., 2008). By increasing lipolysis and free fatty acids in adipocytes, insulin resistance leads to an influx of free fatty acids into the liver. This inhibits insulin-mediated suppression of very low density lipid secretion, which is crucial for preventing post-prandial hyperlipidemia and for maintaining an optimal lipid balance (Kamagate et al., 2008). Insulin resistance thus results in higher and more prolonged post-prandial very low density and other lipids, a process that may represent yet another pathway by which insulin potentially exacerbates pathological Aβ processes in the brain. Despite these findings, the connection between cholesterol and dementia is complex. High plasma cholesterol at midlife is associated with higher Aβ40 levels (Smith and Betteridge, 2004) and a 2–3 fold increased risk for later Alzheimer’s disease dementia (Anstey et al., 2008). Late-life total cholesterol, however, does not appear to be associated with an increased Alzheimer’s disease risk (Anstey et al., 2008), and may in fact be protective to some degree (Cedazo-Minguez et al., 2011; Reitz et al., 2005).
4.3 Vascular impairment and hypertension
Insulin dysfunction can substantially impact the vasculature via direct effects, hyperlipidemia, and inflammation. Insulin plays a modulatory role in capillary recruitment, vasodilation, and regional blood flow (Cersosimo and DeFronzo, 2006; Schinzari et al., 2010), acting to increase vasodilation and regulation of vasoconstriction via modulation of nitric oxide and endothelian-1. Conversely, insulin resistance-associated declines in nitric oxide and increases in endothelin-1 activity results in vasoconstriction and reduced blood flow. This process in turn exacerbates both glucose and lipid dysfunction. As a result, increasing insulin resistance and progressive vascular dysfunction work together in a negative feedback loop (Cersosimo and DeFronzo, 2006).
Fifty percent of hypertensive patients are also insulin resistant (Lima et al., 2009). Hypertension impairs neuron-dependent blood flow (known as functional hyperemia) via a number of insulin-resistance related processes including oxidative stress, dysregulation of vasoactive mediators (including nitric oxide and endothelin-1), structural alteration of the blood vessels, and insufficient cerebral autoregulation (Iadecola and Davisson, 2008). Animal models evidence increased Aβ deposition with hypertension, which leads to vascular dysfunction and reduced functional hyperemia (Iadecola and Davisson, 2008). In population-based studies, hypertension at mid-life is a risk factor for Alzheimer’s disease and vascular dementia, lower brain weight, and Aβ plaque load (Kivipelto et al., 2001; Launer et al., 2000; Ninomiya et al., 2011; Petrovitch et al., 2000). As with total cholesterol, however, studies examining the effects of late-life hypertension on dementia are mixed, and blood pressure may in fact decline in the years prior to and following clinical dementia diagnosis (Kennelly et al., 2009).
4.4 Obesity
Obesity is a growing and dangerous epidemic in the United States and is closely linked to insulin dysregulation; eighty percent of obese individuals are insulin resistant (Cornier et al., 2008). Insulin typically inhibits adipocyte lipase action, which decreases the release of free fatty acids from adipose tissue. With obesity and insulin resistance, however, this process is disturbed and leads to chronically elevated free fatty acids (Cornier et al., 2008). Free fatty acids are linked to Alzheimer’s pathology through a number of potential mechanisms, including inducing inflammation, promoting Aβ deposition, and inhibiting Aβ clearance. Elevated free fatty acids inhibit insulin degrading enzyme, which is both essential for normal insulin signaling and vital for Aβ clearance.(Bravata et al., 2004) Further, free fatty acids promote the development of amyloid and tau filaments in vitro (Axen et al., 2003; Bray et al., 2002). Free fatty acids also induce inflammation, particularly through interactions with tumor necrosis factor-α, an inflammatory cytokine that has been implicated in Alzheimer’s pathogenesis, particularly Aβ accumulation in brain (Proietto et al., 1999; Vessby et al., 2001) (López S, 2008). Tumor necrosis factor-α is over-expressed in adipose tissue of obese animals and humans, whereas neutralization of tumor necrosis factor-α increases insulin sensitivity and decreases plasma free fatty acid levels (Piers et al., 2002).
In assessing associations between obesity and dementia, adiposity (and particularly central adiposity) at midlife carries the highest risk for later dementia. Recent pooled risk estimates of a twofold increase in dementia risk for those with a body mass index in the obese range were reported. Midlife obesity is associated with a risk for dementia later in life over and above its association with other insulin-resistance related conditions (Kivipelto et al., 2005; Luchsinger et al., 2008; Whitmer et al., 2008). Evidence concerning the effects of late-life adiposity on dementia risk is less clear, however (Whitmer et al., 2008), and individuals typically begin to lose weight with the onset of dementia. However, a large Spanish study recently demonstrated that overweight and obese nondemented individuals over the age of 65 performed significantly worse across a range of cognitive measures. Despite conflicting literature in this area, however, it is likely that targeting the obesity epidemic in mid-life or before would have substantial beneficial effects on overall health status and cognitive function.
5. NOVEL PHARMACOLOGICAL STRATEGIES
5.1 Intranasal insulin
One innovative therapeutic strategy currently under investigation is the normalization of brain insulin levels through intranasal insulin administration. As reviewed in the previous sections, insulin has pleiotropic effects on pathways implicated in Alzheimer’s disease pathogenesis. As such, augmenting central nervous system insulin, in contrast to the majority of therapeutic approaches that focus on narrowly defined mechanisms such as acetylcholine modulation or amyloid accumulation, may have greater potential to impact the clinical symptomatology associated with Alzheimer’s disease pathology. Studies administering intravenous insulin while maintaining euglycemia show increased central nervous system insulin and improved cognition (Craft et al., 2003; Craft et al., 1999; Craft et al., 1996; Park et al., 2000). However, chronic peripheral insulin administration is not a viable therapy due to risks associated with hypoglycemia and prolonged peripheral hyperinsulinemia. Any long-term treatment strategy for normalizing central nervous system insulin levels in persons with Alzheimer’s disease must avoid significantly increasing insulin in the periphery. There is increasing support that this may be achieved via intranasal pathways.
The nasal cavity is unique in that olfactory sensory neurons are directly exposed to the external environment in the upper nasal cavity while their axons extend through the cribriform plate to the olfactory bulb. Following intranasal administration, drugs can be directly transported to the central nervous system, bypassing the periphery, via extracellular pathways.(Born et al., 2002; Frey, 2002; Thorne et al., 2004). Additionally, an intraneuronal pathway delivers drugs to the central nervous system hours or days later (Baker and Spencer, 1986; Balin et al., 1986; Broadwell and Balin, 1985; Shipley, 1985). Kern et al.(Kern et al., 1999) administered 40 IU of insulin intranasally in young, healthy adults, resulting in increased cerebrospinal fluid insulin levels within 10 minutes of administration compared to placebo with peak levels noted within 30 minutes. Cerebrospinal fluid insulin levels had not returned to baseline by the end of the 80-minute study. Blood glucose and insulin levels did not change, demonstrating that the effects in cerebrospinal fluid are not due to transport from the nasal cavity to systemic circulation. This finding is consistent with a large literature that demonstrates insulin’s poor transport from the nasal cavity into blood (Illum, 2002). Although elevated cerebrospinal fluid insulin levels do not conclusively demonstrate that brain insulin levels are similarly elevated, animal studies show labeled intranasal insulin uptake to hippocampus and cortex (Francis et al., 2008). In a murine diabetes model, intranasal insulin reduced brain atrophy and neuronal nuclear factor kappa B activation, while increasing synaptic markers, choline acetyltranferase levels, and activation of Akt, cAMP response element binding protein, and glycogen synthase kinase-3β. These effects were accompanied by memory enhancement on water maze and radial arm tasks (Francis et al., 2008).
Human functional and cognitive studies of intranasal insulin also support insulin’s transport to the central nervous system. Intranasal insulin treatment induced changes in auditory-evoked brain potentials compared to placebo (Kern et al., 1999). Several studies have reported that two months of daily insulin administration (4 × 40 international units/day) significantly improves verbal memory and enhanced mood in young healthy adults (Benedict et al., 2004; Benedict et al., 2008; Hallschmid et al., 2008; Stockhorst et al., 2004). In a series of groundbreaking intervention trials, acute and chronic effects of intranasal insulin administration on cognitive function, cerebrospinal fluid biomakers, and functional positron emission tomography imaging were evaluated in memory impaired older adults. Initial pilot data from these studies showed that intranasal improved verbal memory acutely in persons with Alzheimer’s disease or amnestic mild cognitive impairment without affecting plasma insulin or glucose levels, and that 20 international units of insulin produced the greatest benefit (Reger et al., 2006; Reger et al., 2008a). Subsequently, investigation of the chronic effects of 20 international units of intranasal insulin (Reger et al., 2008b) demonstrated that insulin treated subjects had better declarative memory and selective attention performance following 21 days of treatment. In addition, caregivers rated those with greater impairment at baseline as performing significantly better functionally following treatment with insulin, while there was no noted improvement in the placebo group. Fasting plasma insulin and glucose levels were unchanged for both groups, while Aβ40/42 ratios increased for intranasal insulin-treated adults compared to placebo, reflecting increased Aβ40 relative to Aβ42.
A follow up study examined daily intranasal insulin treatment for 4 months in 104 adults with Alzheimer’s disease or amnestic mild cognitive impairment, and compared two doses (10 or 20 international units twice per day vs. placebo) (Craft et al., 2011). Compared with the placebo group, the lower dose of insulin improved delayed memory, and both insulin doses preserved caregiver-rated ability to carry out daily functions. A scale of general cognitive abilities was also preserved by both doses of intranasal insulin. In exploratory analyses, changes in cerebrospinal fluid Aβ42 and tau/Aβ42 ratios were associated with cognitive and functional changes for insulin-treated participants. Participants in this trial also underwent positron emission tomography imaging; compared with placebo-assigned participants, the lower dose insulin group showed reduced progression of hypometabolism in bilateral frontal, right temporal, bilateral occipital, and right precuneus/cuneus regions over the 4-month treatment period. The higher dose insulin group showed even greater treatment effects indicating less hypometabolism progression in most regions and in left parietal cortex.
The above results provide compelling evidence that intranasal insulin may benefit adults with amnestic mild cognitive impairment or Alzheimer’s disease, and thus may represent a promising novel therapeutic approach to the treatment of degenerative memory disorders. Longer, larger, multi-site trials, currently underway in the United States, will address the question of whether intranasal insulin represents a viable therapeutic approach to the treatment of Alzheimer’s disease and mild cognitive impairment.
5.2 Insulin-sensitizing agents: Peroxisome proliferator-activated receptor-γ agonists
Peroxisome proliferator-activated receptor-γ agonists have been shown to improve insulin sensitivity by decreasing circulating insulin and increasing insulin-mediated glucose uptake, with minimal risk of hypoglycemia (Olefsky, 2000). In addition to improving insulin sensitivity, several investigators have reported that peroxisome proliferator-activated receptor-γ activity may reduce both Aβ accumulation and inflammatory reactants and protect against neurotoxicity (Combs et al., 2000; Delerive et al., 2001; Landreth, 2007; Paik et al., 2000). Insulin resistance leads to increased central Aβ levels, peroxisome proliferator-activated receptor-γ agonist therapy may, by decreasing insulin resistance, help to normalize Aβ levels in brain and to improve associated behavioral symptoms. In vitro, treatment with a low dose of the peroxisome proliferator-activated receptor-γ agonist rosiglitazone increased Aβ clearance via upregulation of low density lipoprotein receptor-related protein 1 (Moon et al., 2011). Another peroxisome proliferator-activated receptor-γ agonist, pioglitazone, prevented insulin resistance and related increases in Aβ in fructose-drinking rats (Luo et al., 2011).
Activation of peroxisome proliferator-activated receptor-γ has also been shown to regulate inflammatory responses and apoptosis (Corton et al., 2000; Escher and Wahli, 2000). Peroxisome proliferator-activated receptor-γ agonists inhibit Aβ stimulated secretion of pro-inflammatory products, arrest the evaluation of activated macrophages, and inhibit the expression of cyclooxygenase-2 (Combs et al., 2000). Decreased oxidative stress and reduced inflammation have been demonstrated with peroxisome proliferator-activated receptor-γ treatment in both in vitro and in vivo models (Hirsch et al., 2003; Schmidt et al., 2003). Rosiglitazone suppressed inflammation in brain cells in vitro (Park et al., 2003) and reversed the negative effects of tumor necrosis factor-α on insulin signaling and insulin-mediated glucose uptake (Hernandez et al., 2004). Animal models suggest that treatment with rosiglitazone significantly reduces inflammation in response to acute injury, and also improves endothelium-dependent vasodilation and coronary arteriole function (Bagi et al., 2004; Cuzzocrea et al., 2004; Tao et al., 2003). Treatment with pioglitazone improved cognitive performance and cerebral glucose metabolism, and reduced oxidative stress in rats treated with intracerebroventricular streptozotocin (Pathan et al., 2006). In humans, non-diabetic patients with cardiovascular disease were treated with rosiglitazone and demonstrated notable peripheral anti-inflammatory effects (Sidhu et al., 2003, 2004). In obese non-diabetic patients, rosiglitazone reduced c-reactive protein, plasma monocyte chemoattractant protein-1, serum amyloid A, and tumor necrosis factor-α; reductions of c reactive protein and monocyte chemoattractant protein-1 were also noted in the obese diabetic group (Mohanty et al., 2004). These findings suggest that peroxisome proliferator-activated receptor-γ agonists may protect neural tissue from inflammatory damage, and are thus attractive candidates for the treatment of insulin resistance and inflammation associated with early cognitive decline.
Despite a strong rationale to examine the effectiveness of peroxisome proliferator-activated receptor-γ agonist treatment in persons with Alzheimer’s disease, results from human clinical trials have been mixed. One trial of rosigilitazone in patients with amnestic mild cognitive impairment and early Alzheimer’s disease resulted in improved performance on selective cognitive functions and a more favorable plasma Aβ40/42 ratio (Watson et al., 2005). A larger six-month trial failed to yield overall cognitive benefit; however, improvement was noted in subjects without an apolipoprotein E ε4 allele on a task of general cognitive function at the highest dose (Risner et al., 2006). Subsequent phase three clinical trials, however, failed to show any cognitive improvements in patients with mild to moderate Alzheimer’s disease, regardless of genetic status (Gold et al., 2010; Harrington et al., 2011). Pioglitazone has produced similarly mixed results. Treatment with pioglitazone in patients with both type 2 diabetes and Alzheimer’s disease produced improvement on general cognitive status and declarative verbal memory following six months of treatment, as well as improved regional cerebral blood flow in the parietal lobe (Hanyu et al., 2009; Sato et al., 2011). Follow up data demonstrated that improvements in cognitive function were associated with reduced tumor necrosis factor-α, supporting the anti-inflammatory actions of pioglitazone (Hanyu et al., 2010). However, another trial that was designed to primarily assess the safety of pioglitazone in nondiabetic patients with Alzheimer’s disease failed to show any improvements on secondary outcome cognitive and functional measures (Geldmacher et al., 2011).
The inconsistent results from clinical trials using peroxisome proliferator-activated receptor-γ agonists have led to doubt as to whether these compounds represent effective treatments for memory disorders in older adults. In addition, safety concerns related to the effects of rosiglitazone on cardiovascular functioning and heart failure in diabetic patients have been reported. Doubts have been raised concerning reports of increased cardiovascular events with rosiglitazone treatment; however, such safety concerns may nonetheless limit willingness of physicians to prescribe this compound (Mannucci et al., 2010). Interestingly, a recent in vitro model suggested that a subclinical dose of rosiglitazone may produce more beneficial effects on Aβ clearance than higher doses (Moon et al., 2011). Follow up animal and human studies may help to determine if lower doses may also be accompanied by a better safety/tolerability profile. Further, the above trials all included patients with clinically diagnosed Alzheimer’s disease; however, it is possible that treating insulin resistance prior to the onset of clinically significant dementia (e.g, mild cognitive impairment) may produce more favorable cognitive results. Although a few of these trials included patients with amnestic mild cognitive impairment, to date, none have examined the effects of these medications on people with mild cognitive impairment alone. Finally, larger clinical trials utilizing pioglitazone, which seems to have a more favorable safety profile, may help to illuminate whether peroxisome proliferator-activated receptor-γ agonist treatment improves cognitive decline associated with Alzheimer’s disease pathological processes.
Conclusions
With an aging population and concurrent rise in chronic health conditions comes a rapid escalation in the incidence of dementia. Although notable strides have been made with regard to discerning the pathophysiological processes associated with Alzheimer’s disease and other dementias, pharmacological treatment trials to date have generally produced minimal or disappointing results. Fortunately, many of the processes that underlie the neuropathological features of dementia may be related to risk factors that are responsive to pharmacological interventions, such as insulin resistance and related conditions. Insulin resistance exerts potent negative effects associated with both Alzheimer and vascular pathology. Thus, an important focus will be to identify interventions that effectively target insulin resistance at its early stages, prior to the point at which it exerts its most negative effects on cognition. Innovative therapeutic trials that are currently underway will shed additional light on whether brain insulin resistance can be effectively treated. From a public health perspective, targeting and treating insulin resistance prior to the presence of clinical symptoms may lead to a drastic reduction in the socioeconomic costs associated with cognitive aging.
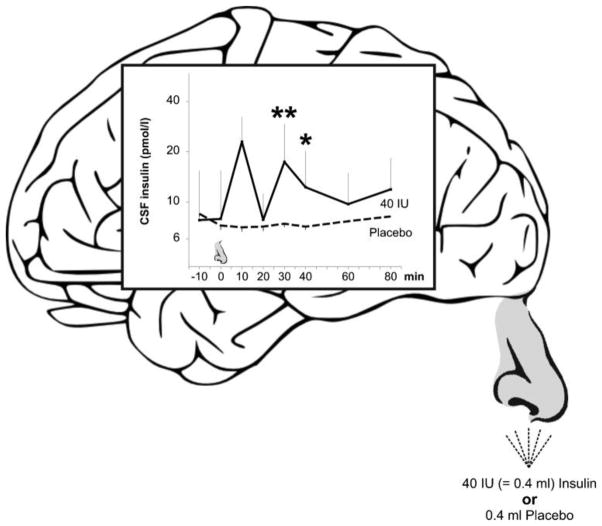
Figure 3.
Sniffing insulin increases cerebrospinal fluid insulin concentrations in humans (Born et al., 2002). Concentrations of insulin in cerebrospinal fluid before and within 80 min after intranasal administration of human insulin (40 international units; solid lines, n=8) and placebo (dashed lines, n=5) Substances were administered with a nasal spray atomizer. Nose symbol indicates time of substance administration. Means ± standard errors are indicated. **P value smaller than 0.01, *P value smaller than 0.05, for pairwise comparisons of baseline adjusted values between both conditions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott MA, Wells DG, Fallon JR. The insulin receptor tyrosine kinase substrate p58/53 and the insulin receptor are components of CNS synapses. J Neurosci. 1999;19:7300–7308. doi: 10.1523/JNEUROSCI.19-17-07300.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. 2008;16:343–354. doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
- Apelt J, Mehlhorn G, Schliebs R. Insulin-sensitive GLUT4 glucose transporters are colocalized with GLUT3-expressing cells and demonstrate a chemically distinct neuron-specific localization in rat brain. J Neurosci Res. 1999;57:693–705. [PubMed] [Google Scholar]
- Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- Authier F, Posner BI, Bergeron JJ. Insulin-degrading enzyme. Clin Invest Med. 1996;19:149–160. [PubMed] [Google Scholar]
- Axen KV, Dikeakos A, Sclafani A. High dietary fat promotes syndrome X in nonobese rats. J Nutr. 2003;133:2244–2249. doi: 10.1093/jn/133.7.2244. [DOI] [PubMed] [Google Scholar]
- Bagi Z, Koller A, Kaley G. PPAR{gamma} activation, by reducing oxidative stress, increases NO bioavailability in coronary arterioles of mice with Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2004;286:H742–H748. doi: 10.1152/ajpheart.00718.2003. [DOI] [PubMed] [Google Scholar]
- Baker H, Spencer RF. Transneuronal transport of peroxidase-conjugated wheat germ agglutinin (WGA-HRP) from the olfactory epithelium to the brain of the adult rat. Exp Brain Res. 1986;63:461–473. doi: 10.1007/BF00237470. [DOI] [PubMed] [Google Scholar]
- Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Archives of neurology. 2011;68:51–57. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balin BJ, Broadwell RD, Salcman M, el-Kalliny M. Avenues for entry of peripherally administered protein to the central nervous system in mouse, rat, and squirrel monkey. J Comp Neurol. 1986;251:260–280. doi: 10.1002/cne.902510209. [DOI] [PubMed] [Google Scholar]
- Banks WA, Jaspan JB, Huang W, Kastin AJ. Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides. 1997a;18:1423–1429. doi: 10.1016/s0196-9781(97)00231-3. [DOI] [PubMed] [Google Scholar]
- Banks WA, Jaspan JB, Kastin AJ. Selective, physiological transport of insulin across the blood-brain barrier: novel demonstration by species-specific radioimmunoassays. Peptides. 1997b;18:1257–1262. doi: 10.1016/s0196-9781(97)00198-8. [DOI] [PubMed] [Google Scholar]
- Baskin DG, Figlewicz DP, Woods SC, Porte D, Jr, Dorsa DM. Insulin in the brain. Annu Rev Physiol. 1987;49:335–347. doi: 10.1146/annurev.ph.49.030187.002003. [DOI] [PubMed] [Google Scholar]
- Baura GD, Foster DM, Porte D, Jr, Kahn SE, Bergman RN, Cobelli C, Schwartz MW. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo. A mechanism for regulated insulin delivery to the brain. J Clin Invest. 1993;92:1824–1830. doi: 10.1172/JCI116773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeri MS, Schmeidler J, Silverman JM, Gandy S, Wysocki M, Hannigan CM, Purohit DP, Lesser G, Grossman HT, Haroutunian V. Insulin in combination with other diabetes medication is associated with less Alzheimer neuropathology. Neurology. 2008;71:750–757. doi: 10.1212/01.wnl.0000324925.95210.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. The Journal of clinical endocrinology and metabolism. 2008;93:1339–1344. doi: 10.1210/jc.2007-2606. [DOI] [PubMed] [Google Scholar]
- Bingham EM, Hopkins D, Smith D, Pernet A, Hallett W, Reed L, Marsden PK, Amiel SA. The role of insulin in human brain glucose metabolism: an 18fluoro-deoxyglucose positron emission tomography study. Diabetes. 2002;51:3384–3390. doi: 10.2337/diabetes.51.12.3384. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nature neuroscience. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Bravata DM, Wells CK, Concato J, Kernan WN, Brass LM, Gulanski BI. Two measures of insulin sensitivity provided similar information in a U.S. population. J Clin Epidemiol. 2004;57:1214–1217. doi: 10.1016/j.jclinepi.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Bray GA, Lovejoy JC, Smith SR, DeLany JP, Lefevre M, Hwang D, Ryan DH, York DA. The influence of different fats and fatty acids on obesity, insulin resistance and inflammation. J Nutr. 2002;132:2488–2491. doi: 10.1093/jn/132.9.2488. [DOI] [PubMed] [Google Scholar]
- Broadwell RD, Balin BJ. Endocytic and exocytic pathways of the neuronal secretory process and trans-synaptic transfer of wheat germ agglutinin-horseradish peroxidase in vivo. J Comp Neurol. 1985;242:632–650. doi: 10.1002/cne.902420410. [DOI] [PubMed] [Google Scholar]
- Burgess BL, McIsaac SA, Naus KE, Chan JY, Tansley GH, Yang J, Miao F, Ross CJ, van Eck M, Hayden MR, van Nostrand W, St George-Hyslop P, Westaway D, Wellington CL. Elevated plasma triglyceride levels precede amyloid deposition in Alzheimer’s disease mouse models with abundant A beta in plasma. Neurobiol Dis. 2006;24:114–127. doi: 10.1016/j.nbd.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Cao D, Lu H, Lewis TL, Li L. Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J Biol Chem. 2007;282:36275–36282. doi: 10.1074/jbc.M703561200. [DOI] [PubMed] [Google Scholar]
- Cedazo-Minguez A, Ismail MA, Mateos L. Plasma cholesterol and risk for late-onset Alzheimer’s disease. Expert Rev Neurother. 2011;11:495–498. doi: 10.1586/ern.11.36. [DOI] [PubMed] [Google Scholar]
- Cersosimo E, DeFronzo RA. Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes Metab Res Rev. 2006;22:423–436. doi: 10.1002/dmrr.634. [DOI] [PubMed] [Google Scholar]
- Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer’s disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J Neurosci. 2000;20:558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DG, Leverenz JB, McMillan PJ, Kulstad JJ, Ericksen S, Roth RA, Schellenberg GD, Jin LW, Kovacina KS, Craft S. Reduced hippocampal insulin-degrading enzyme in late-onset Alzheimer’s disease is associated with the apolipoprotein E-epsilon4 allele. Am J Pathol. 2003;162:313–319. doi: 10.1016/s0002-9440(10)63822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corton JC, Anderson SP, Stauber A. Central role of peroxisome proliferator-activated receptors in the actions of peroxisome proliferators. Annu Rev Pharmacol Toxicol. 2000;40:491–518. doi: 10.1146/annurev.pharmtox.40.1.491. [DOI] [PubMed] [Google Scholar]
- Craft S, Asthana S, Cook DG, Baker LD, Cherrier M, Purganan K, Wait C, Petrova A, Latendresse S, Watson GS, Newcomer JW, Schellenberg GD, Krohn AJ. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer’s disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28:809–822. doi: 10.1016/s0306-4530(02)00087-2. [DOI] [PubMed] [Google Scholar]
- Craft S, Asthana S, Newcomer JW, Wilkinson CW, Matos IT, Baker LD, Cherrier M, Lofgreen C, Latendresse S, Petrova A, Plymate S, Raskind M, Grimwood K, Veith RC. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch Gen Psychiatry. 1999;56:1135–1140. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal Insulin Therapy for Alzheimer Disease and Amnestic Mild Cognitive Impairment: A Pilot Clinical Trial. Archives of neurology. 2011 doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, Luby J, Dagogo-Jack A, Alderson A. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol Aging. 1996;17:123–130. doi: 10.1016/0197-4580(95)02002-0. [DOI] [PubMed] [Google Scholar]
- Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D., Jr Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–168. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3:169–178. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Maffia P, Patel NS, Di Paola R, Ialenti A, Genovese T, Chatterjee PK, Di Rosa M, Caputi AP, Thiemermann C. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-gamma, reduces acute inflammation. Eur J Pharmacol. 2004;483:79–93. doi: 10.1016/j.ejphar.2003.10.056. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao WQ, Ferreira ST, Klein WL. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci U S A. 2009;106:1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Wands JR. Alzheimer’s disease is type 3 diabetes-evidence reviewed. Journal of diabetes science and technology. 2008;2:1101–1113. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delerive P, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol. 2001;169:453–459. doi: 10.1677/joe.0.1690453. [DOI] [PubMed] [Google Scholar]
- Escher P, Wahli W. Peroxisome proliferator-activated receptors: insight into multiple cellular functions. Mutat Res. 2000;448:121–138. doi: 10.1016/s0027-5107(99)00231-6. [DOI] [PubMed] [Google Scholar]
- Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Szot P, Israel PA, Payne C, Dorsa DM. Insulin reduces norepinephrine transporter mRNA in vivo in rat locus coeruleus. Brain research. 1993;602:161–164. doi: 10.1016/0006-8993(93)90258-o. [DOI] [PubMed] [Google Scholar]
- Francis GJ, Martinez JA, Liu WQ, Xu K, Ayer A, Fine J, Tuor UI, Glazner G, Hanson LR, Frey WH, 2nd, Toth C. Intranasal insulin prevents cognitive decline, cerebral atrophy and white matter changes in murine type I diabetic encephalopathy. Brain. 2008;131:3311–3334. doi: 10.1093/brain/awn288. [DOI] [PubMed] [Google Scholar]
- Frey WH., 2nd Intranasal delivery: bypassing the blood-brain barrier to deliver therapeutic agents to the brain and spinal cord. Drug Deliv Technol. 2002;2:46–49. [Google Scholar]
- Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Turk A, Hoyer S, Zochling R, Boissl KW, Jellinger K, Riederer P. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm. 1998;105:423–438. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- Gasparini L, Gouras GK, Wang R, Gross RS, Beal MF, Greengard P, Xu H. Stimulation of beta-amyloid precursor protein trafficking by insulin reduces intraneuronal beta-amyloid and requires mitogen-activated protein kinase signaling. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:2561–2570. doi: 10.1523/JNEUROSCI.21-08-02561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldmacher DS, Fritsch T, McClendon MJ, Landreth G. A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Archives of neurology. 2011;68:45–50. doi: 10.1001/archneurol.2010.229. [DOI] [PubMed] [Google Scholar]
- Gerozissis K, Orosco M, Rouch C, Nicolaidis S. Basal and hyperinsulinemia-induced immunoreactive hypothalamic insulin changes in lean and genetically obese Zucker rats revealed by microdialysis. Brain Res. 1993;611:258–263. doi: 10.1016/0006-8993(93)90511-k. [DOI] [PubMed] [Google Scholar]
- Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, Craft S, Landreth G, Linnamagi U, Sawchak S. Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord. 2010;30:131–146. doi: 10.1159/000318845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallschmid M, Benedict C, Schultes B, Born J, Kern W. Obese men respond to cognitive but not to catabolic brain insulin signaling. International journal of obesity. 2008;32:275–282. doi: 10.1038/sj.ijo.0803722. [DOI] [PubMed] [Google Scholar]
- Hanyu H, Sato T, Kiuchi A, Sakurai H, Iwamoto T. Pioglitazone improved cognition in a pilot study on patients with Alzheimer’s disease and mild cognitive impairment with diabetes mellitus. J Am Geriatr Soc. 2009;57:177–179. doi: 10.1111/j.1532-5415.2009.02067.x. [DOI] [PubMed] [Google Scholar]
- Hanyu H, Sato T, Sakurai H, Iwamoto T. The role of tumor necrosis factor-alpha in cognitive improvement after peroxisome proliferator-activator receptor gamma agonist pioglitazone treatment in Alzheimer’s disease. J Am Geriatr Soc. 2010;58:1000–1001. doi: 10.1111/j.1532-5415.2010.02841.x. [DOI] [PubMed] [Google Scholar]
- Harrington C, Sawchak S, Chiang C, Davies J, Donovan C, Saunders AM, Irizarry M, Jeter B, Zvartau-Hind M, van Dyck CH, Gold M. Rosiglitazone does not improve cognition or global function when used as adjunctive therapy to AChE inhibitors in mild-to-moderate Alzheimer’s disease: two phase 3 studies. Curr Alzheimer Res. 2011;8:592–606. doi: 10.2174/156720511796391935. [DOI] [PubMed] [Google Scholar]
- Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978a;272:827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- Havrankova J, Schmechel D, Roth J, Brownstein M. Identification of insulin in rat brain. Proc Natl Acad Sci U S A. 1978b;75:5737–5741. doi: 10.1073/pnas.75.11.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez R, Teruel T, De Alvaro C, Lorenzo M. Rosiglitazone ameliorates insulin resistance in brown adipocytes of Wistar rats by impairing TNF-alpha induction of p38 and p42/p44 mitogen-activated protein kinases. Diabetologia. 2004 doi: 10.1007/s00125-004-1503-7. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PP. The role of glial reaction and inflammation in Parkinson’s disease. Ann N Y Acad Sci. 2003;991:214–228. doi: 10.1111/j.1749-6632.2003.tb07478.x. [DOI] [PubMed] [Google Scholar]
- Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G, Rocher A, Mobbs CV, Hof PR, Pasinetti GM. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. Faseb J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- Hong M, Lee V. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. Journal of Biological Chemistry. 1997;272:19547–19553. doi: 10.1074/jbc.272.31.19547. [DOI] [PubMed] [Google Scholar]
- Hoyer S. The aging brain. Changes in the neuronal insulin/insulin receptor signal transduction cascade trigger late-onset sporadic Alzheimer disease (SAD). A mini-review. J Neural Transm. 2002;109:991–1002. doi: 10.1007/s007020200082. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illum L. Nasal drug delivery: new developments and strategies. Drug Discov Today. 2002;7:1184–1189. doi: 10.1016/s1359-6446(02)02529-1. [DOI] [PubMed] [Google Scholar]
- Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000;49:1525–1533. doi: 10.2337/diabetes.49.9.1525. [DOI] [PubMed] [Google Scholar]
- Kamagate A, Qu S, Perdomo G, Su D, Kim DH, Slusher S, Meseck M, Dong HH. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest. 2008;118:2347–2364. doi: 10.1172/JCI32914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna H, Hoeft F, Kelley R, Wroolie T, DeMuth B, Reiss A, Rasgon N. Fasting plasma insulin and the default mode network in women at risk for Alzheimer’s disease. Neurobiology of aging. 2013;34:641–649. doi: 10.1016/j.neurobiolaging.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and the risk for dementia: a double edged sword. Ageing Res Rev. 2009;8:61–70. doi: 10.1016/j.arr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Kern W, Born J, Schreiber H, Fehm HL. Central nervous system effects of intranasally administered insulin during euglycemia in men. Diabetes. 1999;48:557–563. doi: 10.2337/diabetes.48.3.557. [DOI] [PubMed] [Google Scholar]
- Kern W, Peters A, Fruehwald-Schultes B, Deininger E, Born J, Fehm HL. Improving influence of insulin on cognitive functions in humans. Neuroendocrinology. 2001;74:270–280. doi: 10.1159/000054694. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Archives of neurology. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- Kopf SR, Baratti CM. Effects of posttraining administration of insulin on retention of a habituation response in mice: participation of a central cholinergic mechanism. Neurobiology of learning and memory. 1999;71:50–61. doi: 10.1006/nlme.1998.3831. [DOI] [PubMed] [Google Scholar]
- Kurochkin IV, Goto S. Alzheimer’s beta-amyloid peptide specifically interacts with and is degraded by insulin degrading enzyme. FEBS Lett. 1994;345:33–37. doi: 10.1016/0014-5793(94)00387-4. [DOI] [PubMed] [Google Scholar]
- Landreth G. Therapeutic use of agonists of the nuclear receptor PPARgamma in Alzheimer’s disease. Curr Alzheimer Res. 2007;4:159–164. doi: 10.2174/156720507780362092. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiology of aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- Lee CC, Kuo YM, Huang CC, Hsu KS. Insulin rescues amyloid beta-induced impairment of hippocampal long-term potentiation. Neurobiol Aging. 2009;30:377–387. doi: 10.1016/j.neurobiolaging.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O’Brien PC, Palumbo PJ. The risk of dementia among persons with diabetes mellitus: a population-based cohort study. Ann N Y Acad Sci. 1997;826:422–427. doi: 10.1111/j.1749-6632.1997.tb48496.x. [DOI] [PubMed] [Google Scholar]
- Lima NK, Abbasi F, Lamendola C, Reaven GM. Prevalence of insulin resistance and related risk factors for cardiovascular disease in patients with essential hypertension. Am J Hypertens. 2009;22:106–111. doi: 10.1038/ajh.2008.263. [DOI] [PubMed] [Google Scholar]
- López SBB, Pacheco YM, Villar J, Abia R, Muriana FJ. Distinctive postprandial modulation of beta cell function and insulin sensitivity by dietary fats: monounsaturated compared with saturated fatty acids. American Journal of Clinical Nutrition. 2008;88:638–644. doi: 10.1093/ajcn/88.3.638. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA. Adiposity, hyperinsulinemia, diabetes and Alzheimer’s disease: an epidemiological perspective. Eur J Pharmacol. 2008;585:119–129. doi: 10.1016/j.ejphar.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R. Body mass index, dementia, and mortality in the elderly. J Nutr Health Aging. 2008;12:127–131. doi: 10.1007/BF02982565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Hou X, Hou L, Wang M, Xu S, Dong C, Liu X. Effect of pioglitazone on altered expression of Abeta metabolism-associated molecules in the brain of fructose-drinking rats, a rodent model of insulin resistance. Eur J Pharmacol. 2011;664:14–19. doi: 10.1016/j.ejphar.2011.04.045. [DOI] [PubMed] [Google Scholar]
- Maher PA, Schubert DR. Metabolic links between diabetes and Alzheimer’s disease. Expert Rev Neurother. 2009;9:617–630. doi: 10.1586/ern.09.18. [DOI] [PubMed] [Google Scholar]
- Mamo JC, Jian L, James AP, Flicker L, Esselmann H, Wiltfang J. Plasma lipoprotein beta-amyloid in subjects with Alzheimer’s disease or mild cognitive impairment. Ann Clin Biochem. 2008;45:395–403. doi: 10.1258/acb.2008.007214. [DOI] [PubMed] [Google Scholar]
- Mannucci E, Monami M, Di Bari M, Lamanna C, Gori F, Gensini GF, Marchionni N. Cardiac safety profile of rosiglitazone: a comprehensive meta-analysis of randomized clinical trials. Int J Cardiol. 2010;143:135–140. doi: 10.1016/j.ijcard.2009.01.064. [DOI] [PubMed] [Google Scholar]
- McDermott JR, Gibson AM. Degradation of Alzheimer’s beta-amyloid protein by human and rat brain peptidases: involvement of insulin-degrading enzyme. Neurochemical Research. 1997;22:49–56. doi: 10.1023/a:1027325304203. [DOI] [PubMed] [Google Scholar]
- Mohanty P, Aljada A, Ghanim H, Hofmeyer D, Tripathy D, Syed T, Al-Haddad W, Dhindsa S, Dandona P. Evidence for a potent antiinflammatory effect of rosiglitazone. J Clin Endocrinol Metab. 2004;89:2728–2735. doi: 10.1210/jc.2003-032103. [DOI] [PubMed] [Google Scholar]
- Moon JH, Kim HJ, Yang AH, Kim HM, Lee BW, Kang ES, Lee HC, Cha BS. The effect of rosiglitazone on LRP1 expression and amyloid beta uptake in human brain microvascular endothelial cells: a possible role of a low-dose thiazolidinedione for dementia treatment. Int J Neuropsychopharmacol. 2011:1–8. doi: 10.1017/S1461145711001611. [DOI] [PubMed] [Google Scholar]
- Ninomiya T, Ohara T, Hirakawa Y, Yoshida D, Doi Y, Hata J, Kanba S, Iwaki T, Kiyohara Y. Midlife and late-life blood pressure and dementia in Japanese elderly: the hisayama study. Hypertension. 2011;58:22–28. doi: 10.1161/HYPERTENSIONAHA.110.163055. [DOI] [PubMed] [Google Scholar]
- Olefsky JM. Treatment of insulin resistance with peroxisome proliferator-activated receptor gamma agonists. J Clin Invest. 2000;106:467–472. doi: 10.1172/JCI10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott A, Stolk RP, Hofman A, van Harskamp F, Grobbee DE, Breteler MM. Association of diabetes mellitus and dementia: the Rotterdam Study. Diabetologia. 1996;39:1392–1397. doi: 10.1007/s001250050588. [DOI] [PubMed] [Google Scholar]
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- Paik JH, Ju JH, Lee JY, Boudreau MD, Hwang DH. Two opposing effects of non-steroidal anti-inflammatory drugs on the expression of the inducible cyclooxygenase. Mediation through different signaling pathways. J Biol Chem. 2000;275:28173–28179. doi: 10.1074/jbc.M002329200. [DOI] [PubMed] [Google Scholar]
- Pandini G, Pace V, Copani A, Squatrito S, Milardi D, Vigneri R. Insulin has multiple antiamyloidogenic effects on human neuronal cells. Endocrinology. 2013;154:375–387. doi: 10.1210/en.2012-1661. [DOI] [PubMed] [Google Scholar]
- Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiology and Behavior. 2000;68:509–514. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- Park EJ, Park SY, Joe EH, Jou I. 15d-PGJ2 and rosiglitazone suppress Janus kinase-STAT inflammatory signaling through induction of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 in glia. J Biol Chem. 2003;278:14747–14752. doi: 10.1074/jbc.M210819200. [DOI] [PubMed] [Google Scholar]
- Pathan AR, Viswanad B, Sonkusare SK, Ramarao P. Chronic administration of pioglitazone attenuates intracerebroventricular streptozotocin induced-memory impairment in rats. Life Sci. 2006;79:2209–2216. doi: 10.1016/j.lfs.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Perez A, Morelli L, Cresto JC, Castano EM. Degradation of soluble amyloid B-peptides 1-40, 1-42, and the Dutch variant 1-40Q by insulin degrading enzyme from Alzheimer disease and control brains. Neurochemical Research. 2000;25:247–255. doi: 10.1023/a:1007527721160. [DOI] [PubMed] [Google Scholar]
- Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, Nelson J, Davis DG, Hardman J, Foley DJ, Launer LJ. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia aging Study. Neurobiology of aging. 2000;21:57–62. doi: 10.1016/s0197-4580(00)00106-8. [DOI] [PubMed] [Google Scholar]
- Piers LS, Walker KZ, Stoney RM, Soares MJ, O’Dea K. The influence of the type of dietary fat on postprandial fat oxidation rates: monounsaturated (olive oil) vs saturated fat (cream) Int J Obes Relat Metab Disord. 2002;26:814–821. doi: 10.1038/sj.ijo.0801993. [DOI] [PubMed] [Google Scholar]
- Piroli GG, Grillo CA, Reznikov LR, Adams S, McEwen BS, Charron MJ, Reagan LP. Corticosterone impairs insulin-stimulated translocation of GLUT4 in the rat hippocampus. Neuroendocrinology. 2007;85:71–80. doi: 10.1159/000101694. [DOI] [PubMed] [Google Scholar]
- Proietto J, Filippis A, Nakhla C, Clark S. Nutrient-induced insulin resistance. Mol Cell Endocrinol. 1999;151:143–149. doi: 10.1016/s0303-7207(99)00050-7. [DOI] [PubMed] [Google Scholar]
- Qiu W, Walsh D, Ye Z, Vekrellis K, Zhang J, Podlisny M, Rosner M, Safavi A, Hersh L, Selkoe D. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. Journal of Biological Chemistry. 1998;273:32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- Reagan LP, Gorovits N, Hoskin EK, Alves SE, Katz EB, Grillo CA, Piroli GG, McEwen BS, Charron MJ. Localization and regulation of GLUTx1 glucose transporter in the hippocampus of streptozotocin diabetic rats. Proc Natl Acad Sci U S A. 2001;98:2820–2825. doi: 10.1073/pnas.051629798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven GM. Insulin resistance in noninsulin-dependent diabetes mellitus. Does it exist and can it be measured? Am J Med. 1983;74:3–17. doi: 10.1016/0002-9343(83)90650-2. [DOI] [PubMed] [Google Scholar]
- Reaven GM. The insulin resistance syndrome. Curr Atheroscler Rep. 2003;5:364–371. doi: 10.1007/s11883-003-0007-0. [DOI] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Frey WH, 2nd, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA, Plymate SR, Schellenberg GD, Cherrier MM, Craft S. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiology of aging. 2006;27:451–458. doi: 10.1016/j.neurobiolaging.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Green PS, Baker LD, Cholerton B, Fishel MA, Plymate SR, Cherrier MM, Schellenberg GD, Frey WH, 2nd, Craft S. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. Journal of Alzheimer’s disease: JAD. 2008a;13:323–331. doi: 10.3233/jad-2008-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JCS, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008b;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- Reitz C, Luchsinger J, Tang MX, Manly J, Mayeux R. Impact of plasma lipids and time on memory performance in healthy elderly without dementia. Neurology. 2005;64:1378–1383. doi: 10.1212/01.WNL.0000158274.31318.3C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, Zvartau-Hind ME, Hosford DA, Roses AD. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J. 2006;6:246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- Rivera E, Goldin A, Fulmer N, Tavares R, Wands J, de la Monte S. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: Link to brain reductions in acetylcholine. J Alzheimer’s Disease. 2005;8:247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR-gamma agonist pioglitazone in mild Alzheimer disease. Neurobiology of aging. 2011;32:1626–1633. doi: 10.1016/j.neurobiolaging.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Schinzari F, Tesauro M, Rovella V, Galli A, Mores N, Porzio O, Lauro D, Cardillo C. Generalized impairment of vasodilator reactivity during hyperinsulinemia in patients with obesity-related metabolic syndrome. Am J Physiol Endocrinol Metab. 2010;299:E947–952. doi: 10.1152/ajpendo.00426.2010. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Moric E, Schmidt M, Sastre M, Feinstein DL, Heneka MT. Anti-inflammatory and antiproliferative actions of PPAR-{gamma} agonists on T lymphocytes derived from MS patients. J Leukoc Biol. 2003 doi: 10.1189/jlb.0803402. [DOI] [PubMed] [Google Scholar]
- Schubert M, Brazil DP, Burks DJ, Kushner JA, Ye J, Flint CL, Farhang-Fallah J, Dikkes P, Warot XM, Rio C, Corfas G, White MF. Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J Neurosci. 2003;23:7084–7092. doi: 10.1523/JNEUROSCI.23-18-07084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, Kondo T, Alber J, Galldiks N, Kustermann E, Arndt S, Jacobs AH, Krone W, Kahn CR, Bruning JC. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci U S A. 2004;101:3100–3105. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Biobehav Rev. 2000;24:855–872. doi: 10.1016/s0149-7634(00)00040-3. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Figlewicz DF, Kahn SE, Baskin DG, Greenwood MR, Porte D., Jr Insulin binding to brain capillaries is reduced in genetically obese, hyperinsulinemic Zucker rats. Peptides. 1990;11:467–472. doi: 10.1016/0196-9781(90)90044-6. [DOI] [PubMed] [Google Scholar]
- Shipley MT. Transport of molecules from nose to brain: transneuronal anterograde and retrograde labeling in the rat olfactory system by wheat germ agglutinin-horseradish peroxidase applied to the nasal epithelium. Brain Res Bull. 1985;15:129–142. doi: 10.1016/0361-9230(85)90129-7. [DOI] [PubMed] [Google Scholar]
- Sidhu JS, Cowan D, Kaski JC. The effects of rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, on markers of endothelial cell activation, C-reactive protein, and fibrinogen levels in non-diabetic coronary artery disease patients. J Am Coll Cardiol. 2003;42:1757–1763. doi: 10.1016/j.jacc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Sidhu JS, Cowan D, Kaski JC. Effects of rosiglitazone on endothelial function in men with coronary artery disease without diabetes mellitus. Am J Cardiol. 2004;94:151–156. doi: 10.1016/j.amjcard.2004.03.051. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MV. Insulin promotes rapid delivery of N-methyl-D- aspartate receptors to the cell surface by exocytosis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3561–3566. doi: 10.1073/pnas.051634698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Betteridge DJ. Plasma beta-amyloid (A beta) 40 concentration, lipid status and age in humans. Neurosci Lett. 2004;367:48–50. doi: 10.1016/j.neulet.2004.05.081. [DOI] [PubMed] [Google Scholar]
- Sonnen JA, Larson EB, Brickell K, Crane PK, Woltjer R, Montine TJ, Craft S. Different patterns of cerebral injury in dementia with or without diabetes. Archives of neurology. 2009;66:315–322. doi: 10.1001/archneurol.2008.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockhorst U, de Fries D, Steingrueber HJ, Scherbaum WA. Insulin and the CNS: effects on food intake, memory, and endocrine parameters and the role of intranasal insulin administration in humans. Physiology & behavior. 2004;83:47–54. doi: 10.1016/j.physbeh.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Strachan MW, Reynolds RM, Frier BM, Mitchell RJ, Price JF. The relationship between type 2 diabetes and dementia. Br Med Bull. 2008;88:131–146. doi: 10.1093/bmb/ldn042. [DOI] [PubMed] [Google Scholar]
- Sudoh S, Frosch MP, Wolf BA. Differential effects of proteases involved in intracellular degradation of amyloid b-protein between detergent-soluble and -insoluble pools in CHO-695 cells. Biochemistry. 2002;41:1091–1099. doi: 10.1021/bi011193l. [DOI] [PubMed] [Google Scholar]
- Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. The Journal of clinical investigation. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L, Liu HR, Gao E, Teng ZP, Lopez BL, Christopher TA, Ma XL, Batinic-Haberle I, Willette RN, Ohlstein EH, Yue TL. Antioxidative, antinitrative, and vasculoprotective effects of a peroxisome proliferator-activated receptor-gamma agonist in hypercholesterolemia. Circulation. 2003;108:2805–2811. doi: 10.1161/01.CIR.0000097003.49585.5E. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Pronk GJ, Padmanabhan V, Frey WH., 2nd Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Tilvis RS, Kahonen-Vare MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol A Biol Sci Med Sci. 2004;59:268–274. doi: 10.1093/gerona/59.3.m268. [DOI] [PubMed] [Google Scholar]
- Tolppanen AM, Lavikainen P, Solomon A, Kivipelto M, Uusitupa M, Soininen H, Hartikainen S. History of Medically Treated Diabetes and Risk of Alzheimer Disease in a Nationwide Case-Control Study. Diabetes care. 2013 doi: 10.2337/dc12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend M, Mehta T, Selkoe DJ. Soluble Abeta inhibits specific signal transduction cascades common to the insulin receptor pathway. J Biol Chem. 2007;282:33305–33312. doi: 10.1074/jbc.M610390200. [DOI] [PubMed] [Google Scholar]
- Unger JW, Livingston JN, Moss AM. Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Prog Neurobiol. 1991;36:343–362. doi: 10.1016/0301-0082(91)90015-s. [DOI] [PubMed] [Google Scholar]
- Vessby B, Unsitupa M, Hermansen K, Riccardi G, Rivellese AA, Tapsell LC, Nalsen C, Berglund L, Louheranta A, Rasmussen BM, Calvert GD, Maffetone A, Pedersen E, Gustafsson IB, Storlien LH. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia. 2001;44:312–319. doi: 10.1007/s001250051620. [DOI] [PubMed] [Google Scholar]
- Wallum BJ, Taborsky GJ, Jr, Porte D, Jr, Figlewicz DP, Jacobson L, Beard JC, Ward WK, Dorsa D. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocrinol Metab. 1987;64:190–194. doi: 10.1210/jcem-64-1-190. [DOI] [PubMed] [Google Scholar]
- Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes. 2009;58:71–77. doi: 10.2337/db08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Teter B, Morihara T, Lim GP, Ambegaokar SS, Ubeda OJ, Frautschy SA, Cole GM. Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: implications for Alzheimer’s disease intervention. J Neurosci. 2004;24:11120–11126. doi: 10.1523/JNEUROSCI.2860-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Chen H, Xu H, Moore E, Meiri N, Quon MJ, Alkon DL. Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. J Biol Chem. 1999;274:34893–34902. doi: 10.1074/jbc.274.49.34893. [DOI] [PubMed] [Google Scholar]