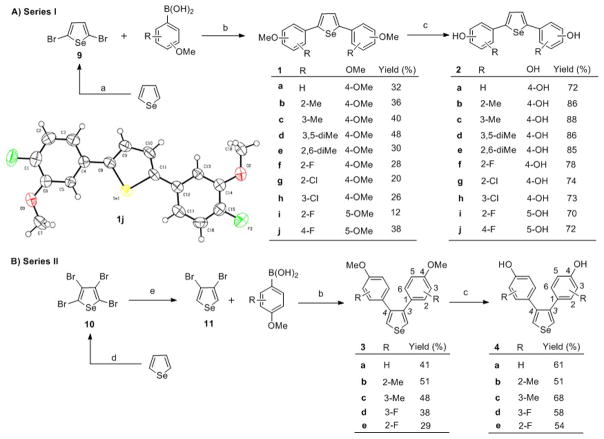
Scheme 1.
Synthesis of 2,5-substituted selenophene compounds. Reagents and conditions: (a) NBS, DMF; (b) [Pd] catalyst (Pd(dppf)Cl2 for Series I; Pd(OAc)2/PPh3 for Series II), Na2CO3, toluene/water (1:1), reflux, 24 h; (c) BBr3, CH2Cl2, −20 °C to rt, 4 h; (d) Br2, AcOH, rt, 18h; (e) Zn, AcOH/water (1:1), 37 °C, 12 h. To simplify comparisons between compounds in closely related series, we designate locant positions of the substituents on the phenyl groups with respect to the selenophene core; for clarity, locant positions on the selenophene core itself are given by numbers in italics.