Table 2.
Comparisons of congeneric selenophene, thiophene and furan-core ER ligands in ERα and ERβ binding affinitiesa.
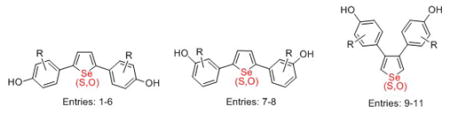
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Selenophenes
|
Thiophenes
|
Furans
|
||||||
| Entry | R | compd | RBA
|
RBA
|
RBA
|
|||
| ERa | ERb | ERa | ERb | ERa | ERb | |||
|
|
|
|
||||||
| 1 | H | 2a | 0.61 ± 0.034 | 2.87 ±0.20 | 0.04 ± 0.004 | 0.79 ± 0.11 | 0.08 ± 0.007 | 0.34 ± 0.10 |
| 2 | 2-Me | 2b | 5.60 ± 0.43 | 11.1 ± 0.73 | 1.43 ± 0.19 | 1.74 ± 0.26 | 0.78 ± 0.12 | 0.24 ± 0.02 |
| 3 | 3-Me | 2c | <0.01 | 0.94 ± 0.05 | <0.01 | <0.01 | -- -- | -- -- |
| 4 | 2-F | 2f | 5.90 ± 0.90 | 24.3 ± 0.52 | 2.03 ± 0.19 | 33.1 ± 5.8 | 6.29 ± 0.73 | 32.2 ± 1.0 |
| 5 | 2-CI | 2g | 6.11 ± 0.049 | 12.7 ± 3.66 | 6.7 ± 1.3 | 10.0 ± 2.4 | 2.73 ± 0.41 | 6.9 ± 1.7 |
| 6 | 3-CI | 2h | 0.29 ± 0.02 | 1.52 ± 0.19 | 0.009 ± 0.001 | 0.036 | -- -- | -- -- |
|
|
|
|||||||
| 7 | 5-F | 2i | 2.02 ± 0.19 | 1.83 ± 0.30 | <0.01 | 0.066 ± 0.006 | -- -- | -- -- |
| 8 | 4-F | 2j | 0.92 ± 0.071 | 3.10 ± 0.73 | <0.01 | 0.010 ± 0.002 | -- -- | -- -- |
|
|
|
|
||||||
| 9 | H | 4a | 0.32 ± 0.04 | 2.01 ± 0.11 | 0.57 ± 0.12 | 1.69 ± 0.45 | 0.22 ± 0.06 | 0.69 ± 0.07 |
| 10 | 2-Me | 4b | 0.27 ± 0.06 | 6.70 ± 0.49 | 2.16 ± 0.54 | 4.9 ± 1.3 | 0.79 ± 0.21 | 2.28 ± 0.34 |
| 11 | 3-Me | 4c | 0.71 ± 0.09 | 0.62 ± 0.12 | 0.830 | 0.297 | -- -- | -- -- |
|
|
|
|
||||||
In each table, the RBA values are given for binding to ERα and ERβ for the various biaryl selenophene, thiophenes and furans we have studied. The disposition of the aryl groups with respect to the heteroatom (Se, S or O) is indicated by the numbers in parentheses.
