Figure 3. CLASP2 is Required for Neural Progenitor Differentiation and Controls Terminal Division of Neural Progenitor Cells.
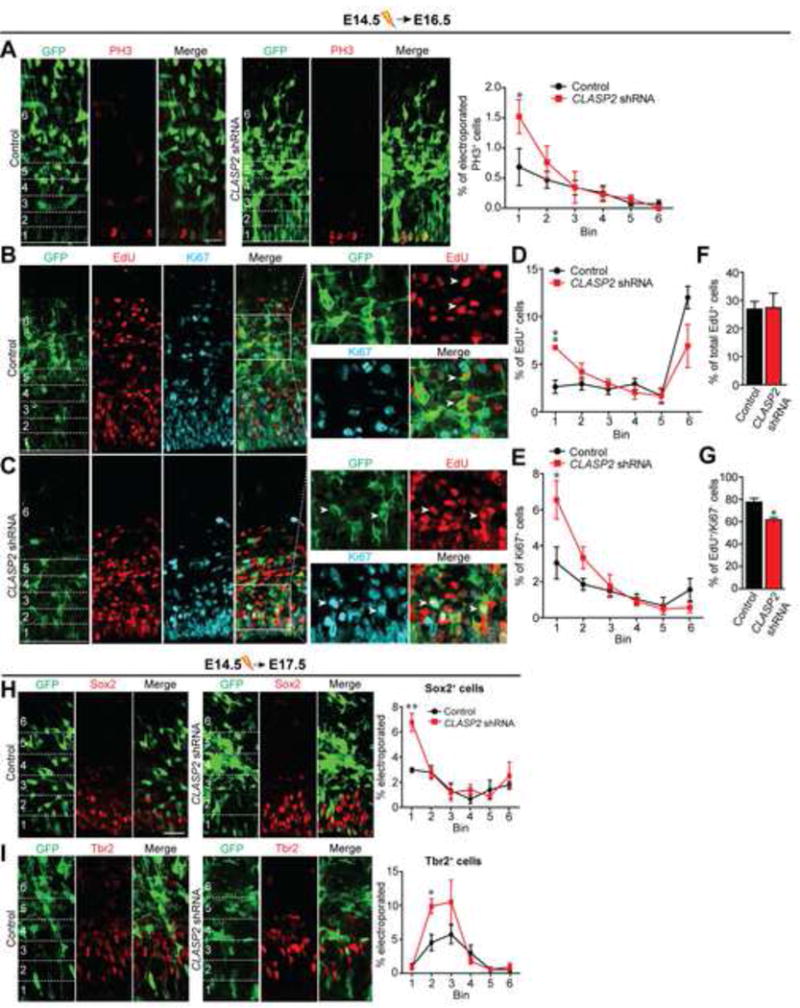
(A) Mouse embryos were electroporated in utero with GFP-tagged scrambled control or CLASP2 shRNA plasmids at E14.5 and analyzed at E16.5. Immunostaining for mitotic marker PH3 (red) shows more dividing cells at the ventricular zone in CLASP2 knockdown cells within the first 20 μm bin (control, n = 4 brains; CLASP2 shRNA, n = 4 brains). For additional data, see Figure S2.
(B–G) Mouse embryos were electroporated in utero with GFP-tagged scrambled control (B) or CLASP2 shRNAs (C) at E14.5. The pregnant dams were injected intraperitoneally with EdU 24 hours following electroporation and embryos were analyzed at E16.5. Coronal brain sections immunostained for EdU (red) and Ki67 (blue) showed an increased in the percentage of electroporated cells which were positive for Edu (D) and Ki67 (E) located in the VZ (bin area 1, within the first 20 μm) in CLASP2 knockdown cells. There was no effect of CLASP2 shRNA on the total number of EdU-positive cells (F); however, there was a significant decrease in the number of EdU-positive/Ki67-negative cells exiting the cell cycle (G). Arrowheads for (B) represents GFP-positive cells which is EdU-positive/Ki67-negative in control brains. Arrowheads for (C) represents GFP-positive cells which is EdU/Ki67-positive in CLASP2 shRNA brains. Control, n = 5 brains and CLASP2 shRNA, n = 4 brains analyzed.
(H–I) Mouse embryos were electroporated in utero with GFP-tagged scrambled control or CLASP2 shRNAs at E14.5 and analyzed at E17.5. Apical and basal progenitors were immunolabeled with antibodies against the transcriptional factors Sox2 (H) and Tbr2 (I). The percentage of transfected cells positive for Sox2 or Tbr2 was calculated every 20 μm bins from the ventricle (control, n = 4 brains; CLASP2 shRNAs, n = 4 brains).
Data are means ± SEM and statistical significance was assessed using one-way ANOVA (*p < 0.05, **p<0.001 for A, D, E, H, I) and unpaired t test (*p < 0.05 for F, G). Scale bar represents 20 μm.
