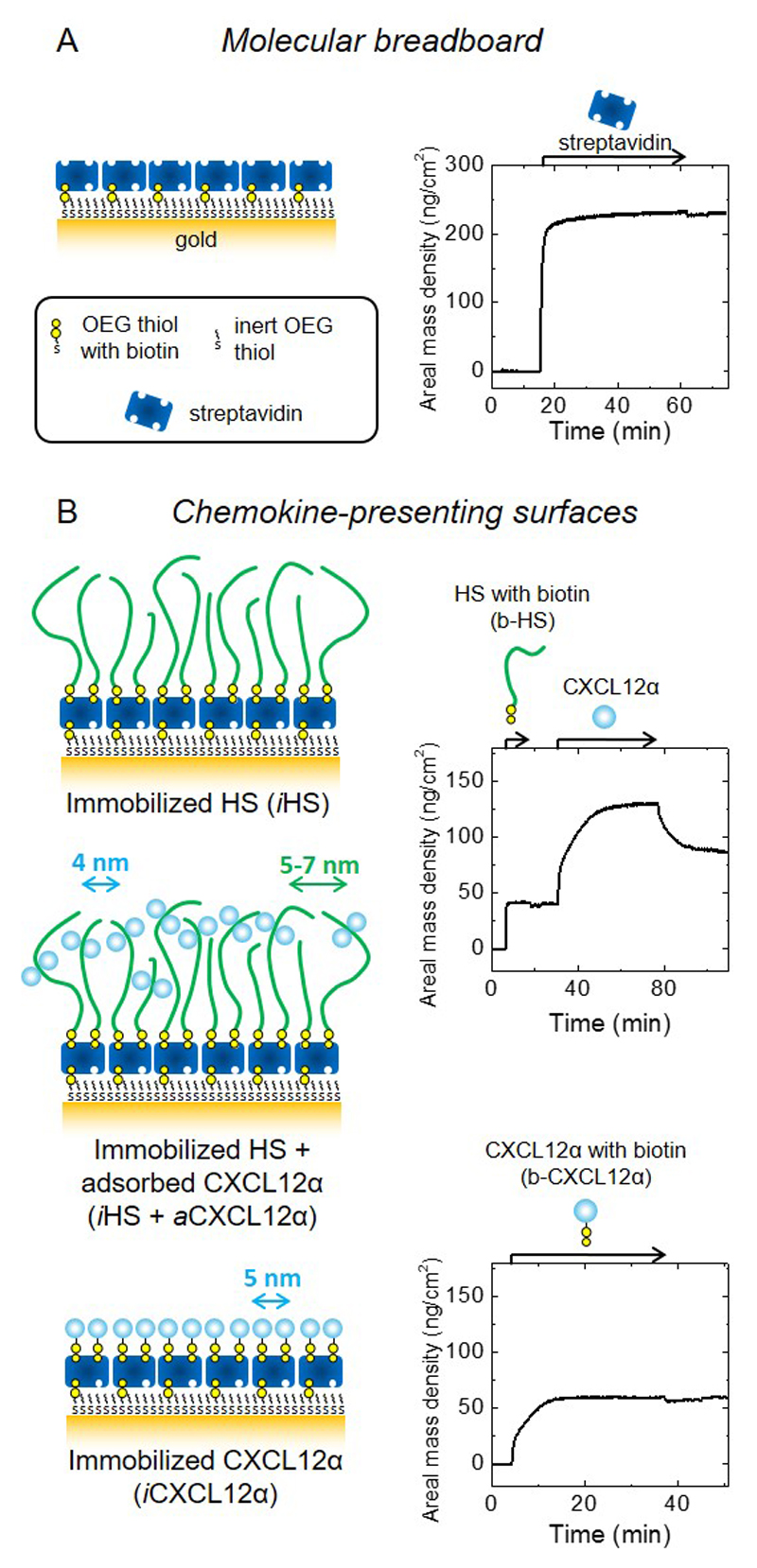
Figure 1. Design and preparation of well-defined biomimetic surfaces presenting GAGs and chemokine.
(A) Schematic presentation of a ‘molecular breadboard’ based on a streptavidin monolayer immobilized on a gold-supported oligoethylene glycol (OEG) monolayer exposing biotin at the end of a fraction of the OEG molecules, where stable attachment to the gold is mediated by thiols. The OEG monolayer (with and without streptavidin) confers a background of low nonspecific binding. (B) Schematic presentation of model surfaces used to study the effect of chemokine presentation on myoblast adhesion and motility: the glycosaminoglycan HS is a native matrix ligand for CXCL12α, and was immobilized (iHS) through a biotin at the reducing end; the chemokine CXCL12α was presented either adsorbed (aCXCL12α) through heparan sulfate (HS) or immobilized (iCXCL12α) through a C-terminal biotin. All molecules are drawn approximately to scale; arrows indicate the lateral root-mean-square (rms) distance between two molecules (colours of molecules and corresponding arrows are matched); iCXCL12α is drawn as monomers but aCXCL12α as dimers, reflecting the known propensity of this chemokine to oligomerize upon HS binding. Streptavidin monolayer formation and the functionalization of the molecular breadboard were followed by spectroscopic ellipsometry (SE) to quantify areal mass densities (A and B, right; see also Table 2). Start and duration of incubation steps with different samples are indicated by arrows on top of the SE graphs; during all other times, the surface was exposed to working buffer.