Supplemental Digital Content is available in the text
Keywords: fecal transplantation, intestinal microbiata, ulcerative colitis
Abstract
Background:
Fecal microbial transplantation (FMT) provides to replace beneficial bacteria with more favorable microbiomes in recipient with dysbiosis. The aim of the present study was to prospectively investigate the efficacy of FMT by assessing the clinical and endoscopic response in patients with ulcerative colitis (UC) who had failed anti-inflammatory and immunosuppressive therapy.
Methods:
In this prospective and uncontrolled study, 30 patients with UC were included. All medications except mesalazine were stopped 4 weeks before FMT. Colonoscopy was performed both before and after FMT. To assess the efficacy of FMT, Mayo scores were calculated at week 0 and week 12. A total of 500 mL extracted fresh fecal suspension was administered into the 30 to 40 cm proximal of terminal ileum of recipients.
Results:
After FMT, 21 of the (70%) 30 patients showed clinical response, and 13 of the 30 (43.3%) patients achieved clinical and endoscopic remission at the week 12. Nine patients (30%) were accepted as a nonresponder at the end of the week 12. There was no significant difference among donors concerning both the rate of clinical remission and clinical response. No adverse events were observed in the majority of patients during FMT and 12 weeks follow-up. Seven patients (23.3%) experienced mild adverse events such as nausea, vomiting, abdominal pain, diarrhea, and fewer after FMT.
Conclusion:
FMT could be considered as a promising rescue treatment modality before surgery in patients with refractory UC. Besides, FMT also appears to be definitely safer and more tolerable than the immunosuppressive therapy in patients with UC (NCT02575040).
1. Introduction
Inflammatory bowel diseases (IBDs), including ulcerative colitis (UC) and Crohn's disease, are a chronic and progressive inflammatory disease of a gastrointestinal tract. It is thought that the primary pathogenic mechanism of IBD is aberrant activation of immune system response to a change in the gut environment.[1] However, the cause of pathologic activation of an innate immune system is not completely understood. In recent years, a growing evidence suggested that endogenous enteric bacteria is also called the intestinal microbiota that may play a pivotal role in the center of IBD pathogenesis. Impaired microbial diversity and intestinal flora species, reduced Firmicutes and Bacteroidetes bacterial phyla, and increased fungi such as Candida have been described in patients with IBD.[2] However, it is unknown whether these alterations are a causative or associative relationship. Microbial dysbiosis and environmental factors are believed to be causally responsible together for development of IBD by triggering immune system in genetically susceptible subjects according to the current knowledge.[3,4]
Apart from the complexity of these pathological mechanisms, treatment of IBD can be difficult in some patients. Standard treatment of IBD includes anti-inflammatory, immunosuppressive agents, and eventually requiring surgery. These options have significant adverse effects. The drug industry has focused on altering immune response by suppressing immune cells rather than addressing the dysbiosis itself in patients with active IBD for a long time. Success of fecal microbial transplantation (FMT) with an overall cure rate of over 80% in treatment of refractory or recurrent Clostridium difficile infection (CDI) led to draw the attention of authors for intestinal diseases including IBD.[5] Unlike the above-mentioned standard therapies, FMT from a healthy donor provides for replacing beneficial bacteria with more favorable microbiomes in recipient with dysbiosis. Therefore, it is not surprising that FMT has raised increasing attention as a rescue therapy approach for patients with IBD through modulating and restoring the balance of gut microbiota.
Although there are a lot of published articles regarding FMT in patients with IBD,[6] a few prospective studies have investigated the role of FMT in the treatment of UC.[7–9] Most of these reports consist of small case series with significant heterogeneity. The aim of the present study was to prospectively investigate the FMT efficacy by assessing the clinical and endoscopic response in patients with UC who had failed anti-inflammatory and immunosuppressive therapy, including inhibitors of tumor necrosis factor (TNF). We also evaluated the adverse events to understand its safety and tolerability.
2. Methods
2.1. Study design
This prospective and uncontrolled study was carried out by the Gastroenterology Department (Gulhane School of Medicine, Ankara, Turkey) as a single center. Patients with UC were enrolled between May 2015 and September 2016. The study protocol was approved by both the institutional ethics committee (KAEK-14045) and Ministry of Health of the Republic of Turkey (56733164/203), in accordance with Declaration of Helsinki. All of the potential risks of FMT (including death and possible necessity surgery despite FMT) were explained, and written informed consent was obtained from all patients and donors before entering. The study was registered on ClinicalTrials.gov (NCT02575040). Each patient has participated in this study for 12 weeks. All authors had access to the study data and had reviewed and approved the final manuscript.
2.2. Study population
In the present study, patients were recruited among 30 individuals who were satisfying the diagnosis of UC criteria according to the confirmation of typical clinical, radiological, endoscopic, and histopathological findings. A detailed history, including smoking status (current smoker and nonsmoker), disease duration and extent, medications, and previously intestinal surgery, was taken from each participant. The extent of disease (proctitis, left-sided colitis, and pancolitis) was determined by Montreal Classification.[10] Patients who have a history of bowel surgery and pregnancy were excluded. The presence of intestinal infectious agents such as C. difficile, Escherichia coli, Salmonella, Shigella, and parasites was investigated by the stool culture and immunoassay methods before the study. Moreover, eligible patients had to be negative regarding HBsAg, anti-hepatitis C virus (HCV), anti-HIV I-II, anti-hepatitis A virus (HAV) IgM, Cytomegalovirus (CMV) IgM, Epstein-Barr virus (EBV) IgM, and syphilis (the venereal disease research laboratory [VDRL]/the traponema pallidum haemagglutination [TPHA]) serologic tests. Venous blood samples were taken for complete blood count, high-sensitivity C-reactive protein (hs-CRP), and sedimentation at the beginning of the study and 12th week. Patients who had used antibiotics and probiotics in the last 4 weeks were also excluded. All patients with UC had used standard immunomodulatory therapies such as the steroid, azathioprine, and anti-TNF before enrollment. Patients with UC who were considered refractory against these conventional therapies (at least 12 weeks for anti-TNF, 4 weeks for the steroid) or steroid-dependent (cannot be tapered less than 10 mg/day within 3 months) were only included as their last chance before surgery. All medications except mesalazine were stopped four weeks before FMT. Besides, all included patients were ≥18 years of age and had active moderate-severe disease based on Mayo Clinic score ≥6 and endoscopic Mayo score ≥2.
2.3. Donor selection
Donated stool for FMT was obtained from partners, relatives, or volunteers (≥18 years of age). Donors were selected from healthy individuals who have no diseases or pathologic conditions potentially associated with changes in gut microbiota. Donors who had used antibiotics and probiotics within the last month were not included for screening. All donors are required to complete a Donor Health Questionnaire form (Suppl 1). To prevent a transmission of donor's infectious disease, all donors were screened for stool tests (stool culture for Salmonella, Shigella, E. coli, Campylobacter, Yersinia, C. difficile by the polymerase chain reaction, and parasites) and serologic tests (i.e., hepatitis A IgM, HBsAg, anti-HCV, anti-HIV I-II, CMV IgM, EBV IgM, syphilis VDRL/TPHA tests). Individuals were not accepted as a donor if they had positive serology for anti-HAV IgM, HBsAg, anti-HCV, and anti-HIV. All donors who met the selection criteria were prospectively rescreened for each donation.
2.4. FMT procedure
Patients have received the bowel lavage (500 mL sennoside a+b calcium and 2 L water) for colonoscopy preparation on the day before FMT. The median amount of 120 to 150 g donor feces was used for FMT preparation. Donors were instructed to collect feces in a small container and to bring it to the hospital on the day of the scheduled transplant. Thus, fresh stool material was used in this study. A total of 500 mL extracted fecal suspension was prepared with 500 mL 0.9% NaCl using the conventional blender and divided into 50 mL syringes. Filtered fecal microbiota suspension was administered into the 30 to 40 cm proximal of terminal ileum of recipients through endoscopic infusion catheter inserted into the colonoscopy under anesthesia. Loperamide was given to all patients to decrease intestinal transit time and to create accurate time for colonization of donor intestinal flora 3 hours before FMT. After the procedure, all patients were under observation for about 6 hours to detect acute adverse effect regarding FMT.
2.5. Clinical outcomes
To assess disease activity and efficacy of FMT, Mayo scores was calculated at week 0 (baseline) and week 12 (primary end point). Therefore, colonoscopy was performed both before and after FMT. The Mayo score that ranges from 0 to 12 point is the total of stool frequency, rectal bleeding, endoscopic findings, and a physician's global assessment (The range of each parameter: 0–3). Endoscopic findings were recorded at 0 and 12 week and were evaluated by an experienced gastroenterologist. The average of last consecutive 3 days was considered as a frequency of rectal bleeding and stool frequency. Clinical response at week 12 was considered as a decrease in the Mayo score ≥30% and ≥3 points when compared with baseline score. Clinical remission was considered as a Mayo score ≤2 points and complete mucosal healing (Mayo endoscopy subscore ≤1). Patients with UC who have achieved clinical remission were also included in the analysis of clinical response. All patients who administered FMT were instructed to contact by telephone or come to the clinic visit regarding with their concerns or side effects. Adverse events were recorded during FMT and 12 weeks follow-up. Association of adverse events with FMT was classified as unrelated, possibly, and definitely related to FMT during the procedure or follow-up period.
2.6. Statistical analysis
The primary end point was assessed on per-protocol basis because all patients who received FMT therapy completed the study procedure. Continuous variables were reported as the mean ± standard deviation, while categorical variables were expressed as frequency and percent (%). The Kolmogorov–Smirnov test was used to determine the distribution characteristics of continuous variables. Wilcoxon signed rank test was performed for paired nonparametric data. The outcomes before and after FMT for paired parametric data were compared using paired t test. The categorical variables between groups were compared by Chi-square or Fisher exact test. Mann–Whitney test was used to find significant differences between nonresponders and responders for nonparametric variables. Logistic regression analysis was performed to determine the association between the rate of FMT success and potential impact factors. Statistical significance was defined as P < 0.05. SPSS (Statistical Package for the Social Sciences ver. 20; SPSS Inc, Chicago, IL) computer program was used for all statistical calculations.
3. Results
3.1. Patient characteristics
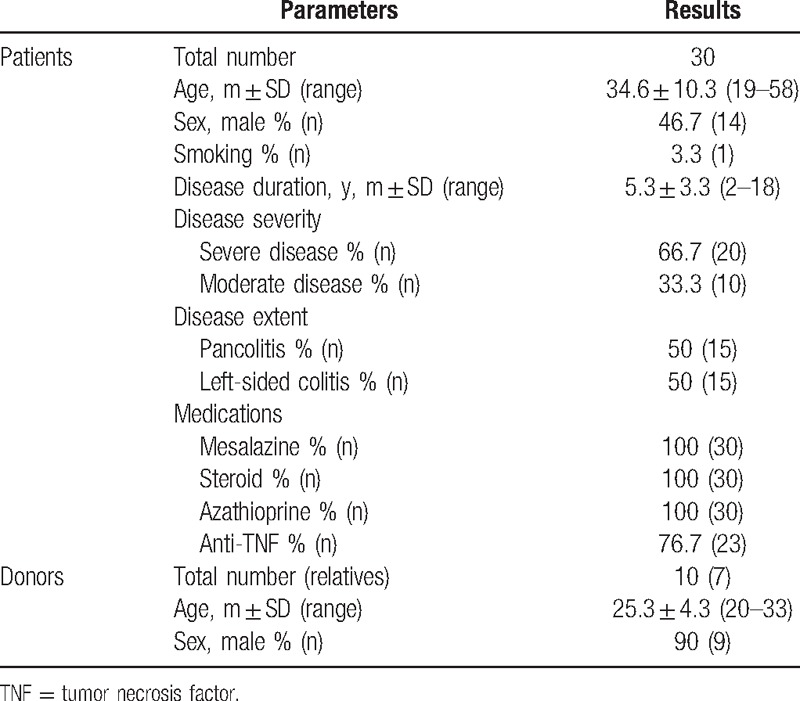
All patients who received FMT completed 12 weeks clinical and endoscopic follow-up. The baseline clinical characteristics of study population before FMT are summarized in Table 1. Patients with UC were 34.6 years of age and 46.7% male. The mean disease duration was 5.3 years (range 2–18 years). Of the patients with UC, 50% (n = 15) had pancolitis and 50% (n = 15) had left-side colitis according to the Montreal classification. Ten patients (33.3%) had moderate UC and 20 patients (66.7%) had severe UC. One patient had sacroiliitis as an extraintestinal manifestation. One patient had a history of breast cancer and one patient had a history of rheumatoid arthritis as an important comorbid medical condition. Twenty-three patients (76.7%) had used all IBD medication options such as 5-ASA, steroid, thiopurines, and anti-TNF before enrolled. In this study, 3 donors were anonymous volunteers and were classified as donor A, B, and C. Donor A, B, and C donated feces for 12 patients, for 7 patients, and for 4 patients, respectively. The remaining 7 patients preferred to have their family members such as mother, partner, and brother as a donor (Donor D). Three recipients received FMT from donor A for a second time because of failure of the first FMT.
Table 1.
The clinical characteristics of study population.
3.2. Outcomes
Clinical remission and response rates were calculated for all subjects at week 12. When the UC patients were categorized into the 2 groups according to the response status as nonresponder and responder groups, there was no significant difference between groups before FMT concerning the baseline clinical and laboratory characteristics of subjects with UC (Table 2). The clinical response was observed in 21 patients with UC (70%). In the responder group, while 13 (43.3%) patients achieved clinical and endoscopic remission at week 12, 8 patients (26.7%) met the criteria of clinical response according to Mayo score (≥30% and ≥3 points decrease from baseline). Nine patients (30%) were accepted as a nonresponder at the end of the week 12. After 12 weeks, while mean hs-CRP, sedimentation and hemoglobin levels were found to be improved significantly compared with the levels before FMT (P = .001, P = .022, and P = .007, respectively), white blood cell (WBC) did not show a significant change (P > .05) (Table 3). Improvements in hs-CRP after FMT were higher in the responder group than in the nonresponder group (P = .038), but there was no significant difference between nonresponder and responder groups regarding improvement in sedimentation and hemoglobin levels (P > .05 for both) (Fig. 1). In addition, we analyzed the correlation between clinical response and clinical characteristics such as age, gender, disease extent, disease duration, and prior medication use. There was no association between clinical response and these potential impact factors (P > .05 for all parameters).
Table 2.
The baseline characteristics of study population before FMT.
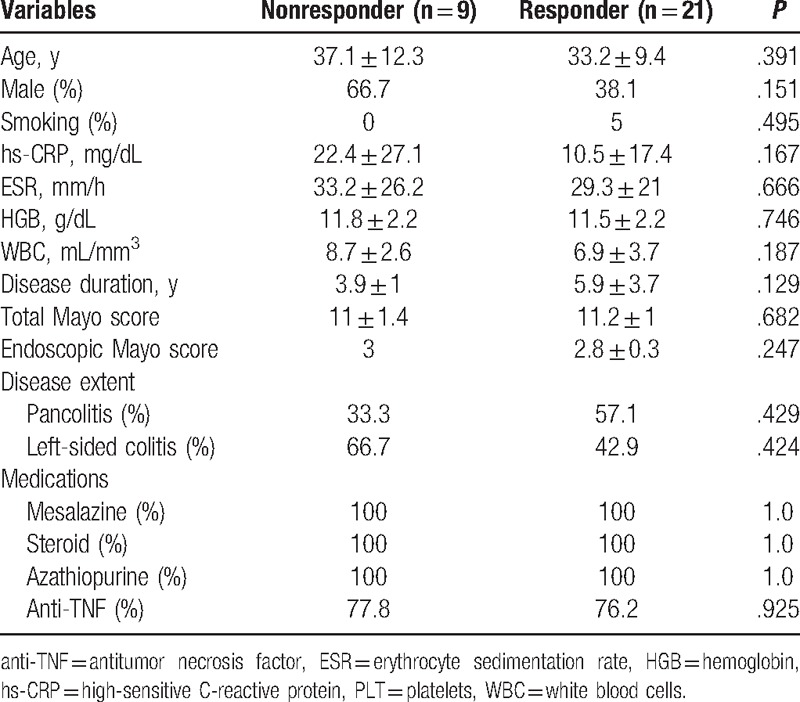
Table 3.
Laboratory parameters and clinical outcomes before and after FMT.
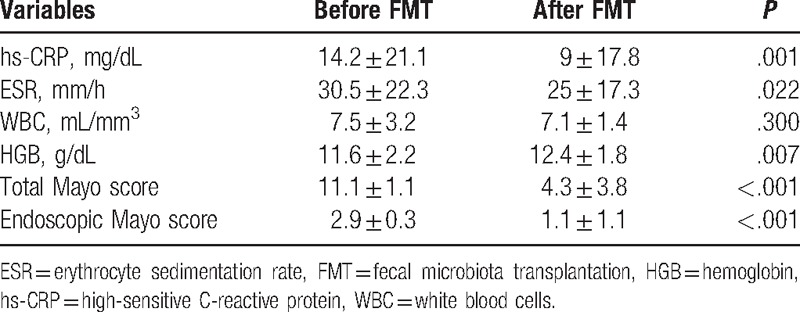
Figure 1.
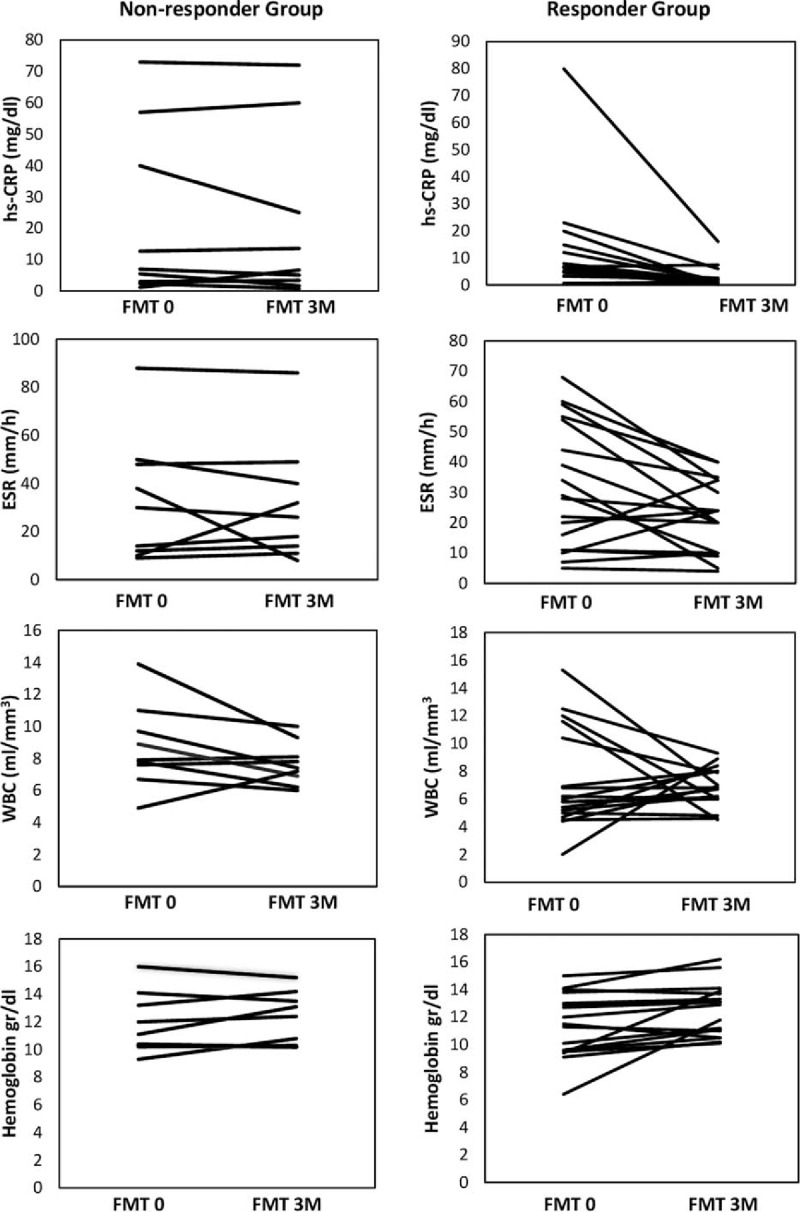
Changes of laboratory parameters after FMT in responder and nonresponder groups. The difference in improvement between groups was significant for hs-CRP, but not for WBC, ESR, and hemoglobin (P = .038 for hs-CRP, P = .328 for WBC, P = .269 for hemoglobin, and P = .198 for ESR).
When FMT success rate was evaluated according to donors, the rate of clinical and endoscopic remission was 50% (6/12) for donor A, 42.9% (3/7) for donor B, 25% (1/4) for donor C, and 42.9% (3/7) for donor D (P > .05). The rate of clinical response was 66.7% (8/12) for donor A, 57.2% (4/7) for donor B, 75% (3/4) for donor C, and 83.8% (6/7) for donor D (P > .05) (Fig. 2). There was no significant difference among donors concerning both the rate of clinical remission and clinical response (P > .05 for all results).
Figure 2.
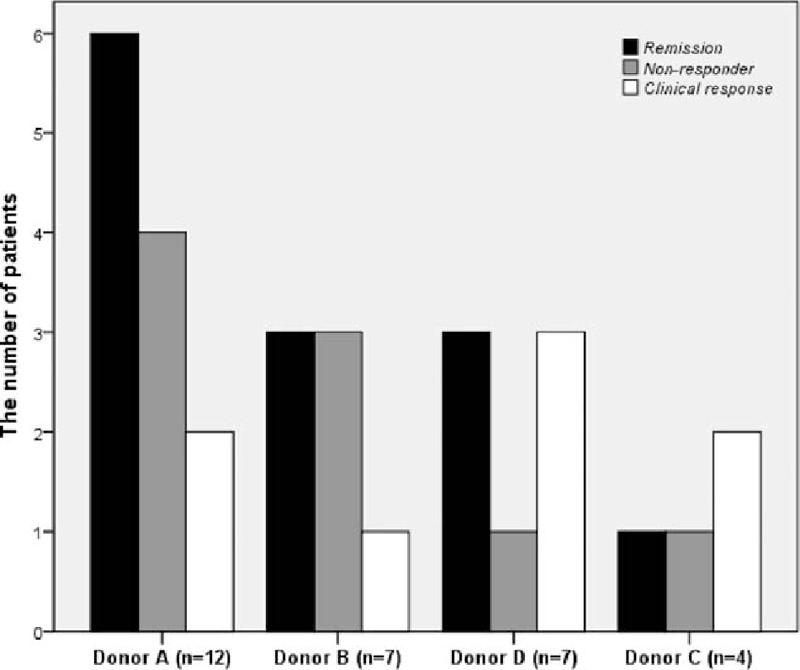
The FMT success rate of each donor according to the Mayo score.
3.3. Safety and tolerability of FMT
No adverse events were observed in the majority of patients during FMT and 12 weeks follow-up. Seven patients (23.3%) experienced mild adverse events such as nausea, vomiting, abdominal pain, diarrhea, and fewer after FMT. These adverse events were short-term and disappeared within 1 day with supportive treatments. These adverse events were accepted as “probably” associated with FMT. Interestingly, all 4 patients who developed a fever after FMT achieved the clinical and endoscopic remission. Infectious complications were not observed. All patients with UC well tolerated FMT. Of patients with UC, 76.6% (23/30) were willing to undergo FMT for UC treatment again regardless of response status in the next time.
4. Discussion
In this prospective and uncontrolled study, we showed that 43.3% of patients with refractory UC achieved clinical, endoscopic, and laboratory remission and 26.7% achieved a clinical response at the end of the week 12 after FMT. We also showed that no significant difference existed among overall donor groups in terms of FMT success rate. In addition, during 12 weeks follow-up, no serious adverse events related FMT was observed and FMT therapy was well tolerated by all patients with UC. By the help of this study, a novel and promising result with the one of the highest clinical responses rate for the treatment of UC has been added to scientific literature as a rescue treatment modality before surgery for patients with refractory UC.
In recent years, the evidence regarding the role of disturbed intestinal microbiota in different disease conditions has begun to accumulate through culture-independent sequencing techniques rapidly.[11] Therefore, the attention of clinicians has been focus on detecting remarkable changes using these advanced techniques in the gut microbiota of patients with UC. The role of each species of intestinal microbiota is not known exactly yet. However, observing beneficial effects after transplantation of all species from the healthy donor is considered as that some species of intestinal microbiota may play role in protection of health while some other species may play a role in development of disease. Clinical studies have shown that several changes in phylum level of gut microbiota composition, including a decrease of Firmicutes and Bacteriodetes and a concomitant increase of Proteobacteria and Actinobacteria, are related to UC.[12] However, it is still debated whether these changes are responsible for activation of the immune system or development of a disease according to the current knowledge. Reestablishment of the microbial diversity by infusion of donor feces was first considered as a treatment option for patients with CDI. Subsequently, the success of FMT in recurrent CDI refractory to standard antibiotic treatment led to an expectation that FMT may re-emerge as a possible therapeutic option for the intestinal diseases including UC. CDI occurs as a result of disruption of gut microbiota after antibiotics,[13] whereas complex pathologic mechanisms such as immunologic, gut microbiota, and genetic play a role in the pathogenesis of UC.[3] Therefore, efficacy rate of FMT in patients with UC may not be as high as in patients with CDI because of different pathologic mechanisms. To date, 3 previous randomized clinical trials with contradictory results were carried out to investigate the efficacy and safety of FMT in patients with UC. Moayyedi et al[7] and Paramsothy et al[8] showed that the rate of clinical remission in patients who received FMT was higher than placebo. In contrast, Rossen et al[9] were not able to demonstrate a significant difference in the rate of clinical remission between the FMT and placebo groups. In a systematic review and meta-analysis, Sun et al[14] reported that the rate of clinical remission in patients with UC was 30.4%. From the data of another recent meta-analysis, Shi et al[15] showed that 40.5% patients with UC achieved clinical remission and 66.1% achieved clinical response. Results obtained by our study were consistent with the latter meta-analysis.
The reason of different success rates of FMT in UC is still unknown. However, it is thought that composition of donor's microbiota may play a role as an important factor influencing the clinical improvement, as this treatment approach is donor dependent. By assessing the results of this study, a difference was seen at the rate of clinical response according to donors, but it was not significant statistically. Small sample size for each donor can explain insignificance of this difference. On the contrary, we identified expanded-criteria donors regarding donor selection. Thus, we think that the reason of the lack of significant difference may depend on close similarity among donor features. In addition, clinical improvement in 3 patients who had failed first FMT was not observed even after second FMT from a different donor. This finding suggests that recipient-related factors may also have influenced the efficacy of FMT. The lack of microbiome composition analysis did not allow for further interpretation in the present study. Therefore, to provide a clearer conclusion, clinical trials with larger sample size and different donor groups should be performed. Another possible explanation for different results in clinical studies is delivery routes and number of FMT infusions. In our study, single-dose FMT was administered for patients with UC except 3 patients who had received FMT from the second donor. Multiple infusions of FMT may increase the rate of clinical remission, especially in patients with clinical response and early recurrences. Cui et al[16] reported that step-up FMT strategy could lead to steroid-free clinical improvement or remission in patients with steroid-dependent UC. However, no difference between multiple and single infusion has been reported by the Sun et al[14] in their subgroup analysis. Therefore, further studies are required to elucidate this procedural aspect.
A variety of routes of administration such as retention enemas, colonoscopy, and nasoduodenal was used for IBD patients in clinical trials. The consensus regarding FMT route has not been established yet. Several clinical trials of FMT for CDI suggest that nasoduodenal route is either associated with higher adverse effects or lower efficacy of FMT because some bacteria species of donor microbiota would not resist the gastric and bile acidity.[17–19] Retention enema may not allow sufficient time for colonization compared with ileal infusion. Our findings, as well as available data, suggest that ileal infusion of feces from healthy donor by the colonoscopy is a safe and tolerable treatment option for patients with UC.[20,21] Moreover, participants had positive attitudes toward FMT in this study. Of the patients with UC, 76.6% were willing to receive the second FMT for UC treatment regardless of response status. Kahn et al[22] have investigated the interest and concerns of patients with UC about FMT. They suggested that most of the patients with UC are quite interested and willing to consider FMT despite the lack of sufficient data regarding safety and efficacy. In the present study, the majority of patients during FMT and 12 weeks of follow-up had no adverse events. Seven patients have experienced self-limited fever or abdominal complaints after FMT. Interestingly, clinical and endoscopic remission was observed in all patients who have developed a fever after FMT without bacteremia. This finding could be explained by the transient systemic immune response during engraftment of donor microbiota. Therefore, we speculate that the fever after FMT may be a good predictor of clinical remission.
Changes in the laboratory parameters are worth deliberating because they show a strong correlation with disease activity, severity, and treatment response.[23] Laboratory parameters including CRP, sedimentation, WBC, and hemoglobin levels are as important as clinical parameters such as rectal bleeding, stool frequency, and endoscopic findings in the global assessment of UC. Changes in hemoglobin levels show the hemodynamic improvement in the patient with UC, while inflammatory markers can be used to predict disease severity, disease activation, and treatment response. In our study, we observed that CRP, hemoglobin, and sedimentation level improved after FMT in both responder and nonresponder groups, but not WBC levels. Furthermore, we also showed that the decreasing CRP levels in patients who benefited from FMT were higher than the patients who failed FMT. This result suggests that CRP may predict the clinical response of FMT. Conversely, no significant difference existed between groups in terms of improvement sedimentation and hemoglobin levels. Consistent with our results, Zhang et al[24] reported that CRP levels are not able to predict the immediate clinical efficacy, but CRP significantly reduced 3 months after FMT in patients who have achieved clinical response. In fact, the post-hoc analyses of SONİC[25] and ACCENT I[26] have shown that CRP levels are a good predictor for maintaining response and remission to anti-TNF therapy in patients with IBD. Therefore, we recommend that CRP level may be used to estimate the clinical efficacy of FMT.
This study has several limitations. First, we did not include a control group. Perhaps randomization could have helped remove the placebo effect of FMT treatment. Second, the microbiome characterization of donor and recipient was not performed because of financial limitations. Therefore, we could not get the answers to the questions such as which bacteria species play a role in the pathogenesis of UC and which species of donor microbiota are more effective in the treatment of UC. Third, this study was carried out for 12 weeks of follow-up. Long-term follow-up studies are required to determine permanent colonization and to latent adverse events.
In conclusion, we proposed that FMT could be considered as a promising rescue treatment modality before surgery in patients with refractory UC. Besides, FMT also appears to be definitely safer and more tolerable than the immunosuppressive therapy in patients with UC. However, FMT procedure needs to be standardized about donor selection, stool preparation, delivery route, and dosing. Therefore, we recommend that more evidence from long-term and randomized controlled clinical studies examining donor and recipient microbiota composition are needed for the use of FMT in clinical practice.
Supplementary Material
Footnotes
Abbreviations: CDI = Clostridium difficile infection, FMT = fecal microbial transplantation, hs-CRP = high-sensitivity C-reactive protein, IBD = Inflammatory bowel disease, TNF = tumor necrosis factor, UC = ulcerative colitis.
Authorship: AU: Study design, data analysis; KO: Data analysis, data interpretation, figures, literature search, and writing; HD and CO: Data collection; IYA: Data analysis; TT: Statistical analysis; and
MG: Literature search, data interpretation.
This research did not receive any specific grant from funding agencies. The authors have no conflicts of interest to disclose.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 2004;126:1504–17. [DOI] [PubMed] [Google Scholar]
- [2].Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology 2014;146:1489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Serban DE. Microbiota in inflammatory bowel disease pathogenesis and therapy: is it all about diet? Nutr Clin Pract 2015;30:760–79. [DOI] [PubMed] [Google Scholar]
- [4].Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med 2011;365:1713–25. [DOI] [PubMed] [Google Scholar]
- [5].Girotra M, Garg S, Anand R, et al. Fecal microbiota transplantation for recurrent Clostridium difficile infection in the elderly: long-term outcomes and microbiota changes. Dig Dis Sci 2016;61:3007–15. [DOI] [PubMed] [Google Scholar]
- [6].Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis 2014;8:1569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015;149:102–9. [DOI] [PubMed] [Google Scholar]
- [8].Paramsothy S, Kamm M, Walsh A, et al. Multi-donor intense faecal microbiota transplantation is an effective treatment for resistant ulcerative colitis: a randomised placebo-controlled trial. J Crohns Colitis 2016;10:S14. [Google Scholar]
- [9].Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 2015;149:110–8. [DOI] [PubMed] [Google Scholar]
- [10].Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19Suppl A:5A–36A. [DOI] [PubMed] [Google Scholar]
- [11].Cammarota G, Pecere S, Ianiro G, et al. Principles of DNA-based gut microbiota assessment and therapeutic efficacy of fecal microbiota transplantation in gastrointestinal diseases. Dig Dis 2016;34:279–85. [DOI] [PubMed] [Google Scholar]
- [12].Kump PK, Gröchenig HP, Lackner S, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis 2013;19:2155–65. [DOI] [PubMed] [Google Scholar]
- [13].Britton RA, Young VB. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology 2014;146:1547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sun D, Li W, Li S, et al. Fecal microbiota transplantation as a novel therapy for ulcerative colitis: a systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shi Y, Dong Y, Huang W, et al. Fecal microbiota transplantation for ulcerative colitis: a systematic review and meta-analysis. PLoS One 2016;11:e0157259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cui B, Li P, Xu L, et al. Step-up fecal microbiota transplantation strategy: a pilot study for steroid-dependent ulcerative colitis. J Transl Med 2015;13:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kassam Z, Lee CH, Yuan Y, et al. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol 2013;108:500–8. [DOI] [PubMed] [Google Scholar]
- [18].Scaldaferri F, Pecere S, Petito V, et al. Efficacy and mechanisms of action of fecal microbiota transplantation in ulcerative colitis: pitfalls and promises from a first meta-analysis. Transplant Proc 2016;48:402–7. [DOI] [PubMed] [Google Scholar]
- [19].Kump P, Högenauer C. Any future for fecal microbiota transplantation as treatment strategy for inflammatory bowel diseases? Dig Dis 2016;34Suppl 1:74–81. [DOI] [PubMed] [Google Scholar]
- [20].Xu L, Zhang T, Cui B, et al. Clinical efficacy maintains patients’ positive attitudes toward fecal microbiota transplantation. Medicine (Baltimore) 2016;95:e4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Damman CJ, Brittnacher MJ, Westerhoff M, et al. Low level engraftment and improvement following a single colonoscopic administration of fecal microbiotato patients with ulcerative colitis. PLoS One 2015;10:e0133925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kahn SA, Vachon A, Rodriquez D, et al. Patient perceptions of fecal microbiota transplantation for ulcerative colitis. Inflamm Bowel Dis 2013;19:1506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014;14:329–42. [DOI] [PubMed] [Google Scholar]
- [24].Zhang T, Cui B, Li P, et al. Short-term surveillance of cytokines and C-reactive protein cannot predict efficacy of fecal microbiota transplantation for ulcerative colitis. PLoS One 2016;11:e0158227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn's disease in the SONIC trial. Gut 2014;63:88–95. [DOI] [PubMed] [Google Scholar]
- [26].Reinisch W, Wang Y, Oddens BJ, et al. C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn's disease: a post-hoc analysis from ACCENT I. Aliment Pharmacol Ther 2012;35:568–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


