Abstract
Complications resulting from impaired fracture healing have major clinical implications on fracture management strategies. Novel concepts taken from developmental biology have driven research strategies towards the elaboration of regenerative approaches that can truly harness the complex cellular events involved in tissue formation and repair. Advances in polymer technology and a better understanding of naturally derived scaffolds have given rise to novel biomaterials with an increasing ability to recapitulate native tissue environments. This coupled with advances in the understanding of stem cell biology and technology has opened new avenues for regenerative strategies with true clinical translatability. These advances have provided the impetus to develop alternative approaches to enhance the fracture repair process. We provide an update on these advances, with a focus on the development of novel biomimetic approaches for bone regeneration and their translational potential.
Keywords: Fracture repair, biomimetic, endochondral ossification, biomaterials, stem cells
Introduction
Bone tissue has a remarkable ability to regenerate without forming fibrous scar tissue, due to complex biological processes that recapitulate bone development. However, even with this incredible capacity for regeneration, both external and pathological factors can affect this regenerative pathway, leading to delayed fracture healing and in some cases fracture non-union.1,2
A non-union is generally defined by the Food and Drug Administration (FDA) as incomplete healing within 9 months, combined with a lack of radiological characteristics associated with fracture healing observed during the final 3 months.3,4 Approximately 10% of all fractures in the United Kingdom result in non-union, with the resulting cost to the National Health Service (NHS) ranging from £7000 to £79,000 per patient.5
There has been an intense drive towards research focusing on the development of strategies to enhance the fracture-healing process in an attempt to reduce the incidence of failure.4,6 This review aims to summarise novel developments in the field of skeletal regeneration, with a focus on emerging research mimicking biological processes that underpin bone tissue repair.
The fracture repair cascade
The biological aspects of skeletal development and healing have been extensively studied. In order to explore advances within the field of skeletal tissue engineering, we first need to understand the complex yet carefully orchestrated process of fracture repair.
Fractures heal through two mechanisms: intramembranous ossification is involved in direct fracture healing and occurs in less than 2% of fractures. It requires rigid fixation with a gap of less than 0.01 mm and begins with the formation of cutting cones near well-defined fracture ends that create longitudinal cavities. Bone is then laid down by osteoblasts bridging the gap and re-establishing the bone’s lamellar structure without the formation of a cartilage callus.1
Most long-bone fractures, however, heal through the process of indirect fracture healing (Figure 1) driven primarily by endochondral ossification (EO), making it a key area of focus for the development of tissue engineering–based regenerative strategies.7–10 Unlike direct fracture healing, the process of indirect fracture repair takes place if micro-motion occurs within an unstable fracture site.1,2
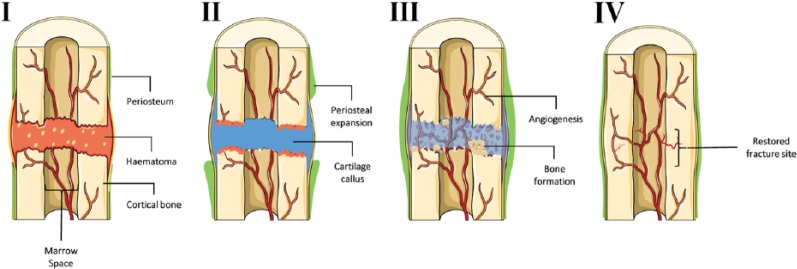
Figure 1.
Stages of endochondral ossification during fracture repair. Stage I – haematoma: initial injury leads to the disruption of surrounding blood vessels resulting in the formation of a platelet-rich fibrin clot. Secreted chemokines promote stem cell expansion and localisation to the fracture site. Stage II – soft callus: prolonged hypoxic conditions within the unstable fracture site favour chondrogenic differentiation of stem cells from the periosteum resulting in a cartilage callus. Stage III – hard callus: chondrocytes within the stabilised callus undergo hypertrophy and eventually apoptosis permitting the invasion of blood vessels and woven bone formation. Stage IV – remodelling: woven bone is remodelled into lamellar bone through the synergistic action of osteoblasts and osteoclasts thus re-establishing native bone physiology. Figure generated using the Servier medical art database (http://www.servier.com/Powerpoint-image-bank) and adapted from Roberts et al.11
There are several key steps to the EO process, as illustrated in Figure 1. Many aspects of EO recapitulate skeletogenesis as observed developmentally. It begins with the initial inflammatory response that leads to the formation of a haematoma, thus laying down a template for callus formation. Although it is known that chronic expression of proinflammatory cytokines have a negative effect on bone, the initial secretion of proinflammatory cytokines triggers the repair process. This early inflammatory response is believed to be initiated by the release of platelet-derived interleukin (IL) 1β,12,13 IL-6,14,15 tumour necrosis factor-α (TNF-α)16,17 and IL-17.13,18,19 These proinflammatory cytokines modulate immune cells and surrounding skeletal stem cell populations.17,18,20–23 The hypoxic conditions within the haematoma lead to an increase in the expression of pro-angiogenic factors thus promoting vascularisation around the fracture site.22,24 A plethora of growth factors including transforming growth factor beta-1 (TGFβ1), fibroblastic growth factors (FGFs), bone morphogenic proteins (BMPs), platelet derived growth factor (PDGF) and stromal-derived factor 1 alpha (SDF1α) are involved in the activation and recruitment of skeletal progenitor cells from the periosteum.23,25–28 It has been suggested that the hypoxic conditions present within the fracture site favour the differentiation of skeletal stem cells towards a chondrogenic phenotype, subsequently producing an avascular cartilage callus.1,24,29 The fracture callus provides stability while chondrocytes within the fracture callus stop proliferating and become hypertrophic.
This is followed by matrix mineralisation, chondrocyte apoptosis and subsequent degradation/resorption of the cartilage matrix.1 Through the actions of osteoclasts and osteoblasts, the mineralised callus is replaced by woven bone. The cortical shell provides stability by bridging the bone ends, allowing for limited weight bearing. The final remodelling stage involves the replacement of woven bone with lamellar bone. Although this process is initiated at 3–4 weeks, its completion can take years depending on the age of the patient.1
The complex biological processes involved in fracture repair can be affected by a number of factors leading to the disruption of bone healing. Some of these factors include the severity of the fracture that may result in surrounding soft tissue damage and compromised vasculature, commonly associated with high impact fractures.30 However, some of the most common factors are host-associated. These include metabolic diseases such as diabetes,31 lifestyle choices such as smoking32 and underlying pathologies such as osteoporosis or the age of the patient which can affect bone quality.33 Any one of these factors, or a combination of many, can result in the failure of this finely balanced process resulting in a delayed or non-union bone fracture.34
Mimicking the fracture repair process as a strategy for non-union repair
As the majority of long-bone fractures heal through the process of EO, which in many respects replicates developmental mechanisms, Lenas and colleagues have defined the concept of ‘developmental engineering’ as a modality for the creation of reparative implants. This states that tissue development progresses through several intermediates, which can be incorporated into a engineered regenerative strategy.35,36 As discussed, EO during fracture repair consists of a number of stages (Figure 1), with a key intermediate stage involving the formation of a cartilage callus (soft callus).37 Understanding the fundamental components that make up the ECM of this tissue is vital in developing substrates capable of partaking in the subsequent stages of EO. Additionally, key regulatory factors that drive cartilage callus formation is vital when considering strategies to mimic its ECM or in priming its progenitor cells.
The ECM is a complex three-dimensional (3D) scaffolding structure particular to each tissue type.38,39 The dynamic and versatile assembly confers the ability of the ECM to modulate the production, degradation and remodelling of its self-assembled macromolecules, thus supporting the development, function and repair of tissues.38–40 The cartilage ECM is predominantly composed of collagen, non-collagenous glycoproteins, hyaluronan and proteoglycans40 (Figure 2). The ECM also maintains a reservoir of growth factors and cytokines that initiate and regulate cell activation and turnover.40–42 Mimicry of natural processes can assist in improving tissue regeneration strategies and it is likely that using a mimetic ECM will help to achieve this by providing niches for cells to reside and form tissues within.
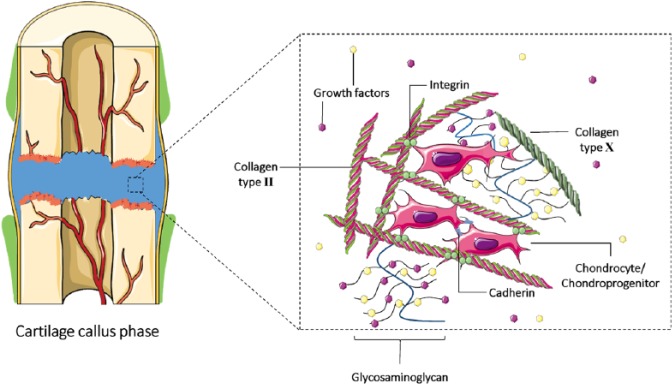
Figure 2.
Potential cell–matrix interactions with the soft/hypertrophic callus. Chondrocytes and chondroprogenitor cells within the fracture cartilage callus extracellular matrix (ECM) may be tethered to collagen type II and X through integrins.40 Cell to cell interactions occur through cadherins. Glycosaminoglycans are bound to the collagen structure and sequester local growth factors within the ECM. Sequestered growth factors interact with chondroprogenitors, activating cell-signalling pathways that promote chondrogenesis, which in turn promotes the expression of matrix remodelling factors.42 Figure generated using the Servier medical art database (http://www.servier.com/Powerpoint-image-bank).
The concepts relating to the creation of these mimetic niches will be further discussed in the context of progress made in synthetic polymer engineering, natural scaffolds and tissue intermediates composed of stem cells and cell-derived matrix.
Synthetic polymer engineering to mimic cell–matrix interactions
Scaffold design and fabrication have been major areas of research as they are key components within tissue engineering and regenerative medicine. The use of polymers is widespread for the fabrication of tissue engineering biomaterials.42–45 Scaffold materials can be synthetic or natural, and non-degradable or degradable depending on their application.45 Natural polymers were some of the first biodegradable biomaterials to be used clinically43 due to their bioactivity contributing to the ability to interact readily with cell populations. Natural polymers include proteins such as silk, gelatin, fibrinogen and collagen, and polysaccharides such as amylose, cellulose, chitin, dextran and glycosaminoglycans.45,46 However, a major limitation of most naturally derived polymers relates to manufacturability and functionalisation. This combined with inconsistent results has led to the investigation of synthetic polymers for tissue engineering purposes. Currently, much research is being focussed on using modified polymers to modulate the interaction of cells with the material in an attempt to replicate cell niches. Major technological advancements in this area include the development of high-throughput screening platforms to define polymer functionalisation and surface topology.
Synthetic polymers such as polyglycolide (PGA), polysulfone (PSU) and polylactic acid (PLA) have been widely investigated in the context of the bone-healing process.47–50 Furthermore, the composition and applications of these polymers has been extensively reviewed.51,52 Despite this, limitations remain pertaining to their limited bioactivity, resulting in restricted cell interactions and tissue-forming capacity.53–55
Several strategies have attempted to overcome some of these hurdles through surface modifications. Through the use of heparin functional groups, growth factors such as basic fibroblast growth factor (bFGF) can be localised to the material surface, resulting in enhanced cell attachment and proliferation.56 The modification of surface topography has also shown great promise in enhancing cell attachment and differentiation, with enhanced osteoblastic cell adhesion on specific surfaces observed through the fabrication of nanoscale topography.57–59 The principle of altering surface topographies has also been applied to the fabrication of PLA/polycaprolactone (PCL) hybrid scaffolds using electrospinning to obtain fibre alignment, demonstrating that this characteristic could enhance chondrogenic differentiation of septum-derived progenitors.60 Other studies have also reported similar success in directing cells towards the chondrogenic fate through the application of surface modifications.61–66 Further modulation of methacrylates demonstrated that the use of functional groups such as phosphates and glycosaminoglycans, which are found within native bone and cartilage, induce hMSCs towards an osteogenic or chondrogenic lineage, respectively.67
The approach of polymer modification offers great promise towards the development of novel biomaterials that can functionally regulate cellular mechanisms. This increasing need for novel polymers has driven research towards screening methodologies such as polymer arrays. The development of polymer array technologies is aimed at the simultaneous screening of several factors or polymer blends, thus downscaling the resources and time needed for the screening process. One such approach involved the development of materials employing varying physical properties or chemical concentrations68,69 (the emergence of polymer array technologies has been extensively reviewed elsewhere70). Some studies have leveraged recent developments in microfabrication through robotic liquid-dispensing technology to examine various conditions. These polymer arrays have been utilised to investigate the control of cell behaviour, including research into pluripotent stem cells,71,72 primary articular chondrocytes73 and for the investigation of pluripotent stem cell interactions when exposed to various glycosaminoglycans and integrins.74 Polymer array technology has also successfully been used to identify specific materials that can aid in the isolation, expansion and differentiation of human skeletal progenitors.75,76 It is envisaged that this technology could be used to find polymers that mimic the complex cell–matrix interactions that are observed during the fracture repair process and thus contribute to engineering the endochondral response. Further screening technologies such as the ‘TopoChip’ utilise unbiased algorithms to fabricate topographies on PLA. This technology successfully allowed for the screening of specific patterns that demonstrate enhanced osteogenic differentiation.77,78 Further optimisation to the system using a chip carrier has also helped eliminate other variations within the culture system that may influence cell viability and adhesion.79 Developments of polymer array technologies show great applicability in the field of bone tissue engineering due to their applicability towards materials, biochemical factors and cell populations currently used within the field. This is especially important due to the emergence of novel innovations in additive fabrication methods such as 3D printing, allowing for the rapid and varied fabrication of material structures. It is therefore essential to utilise robust and high-throughput methodologies for material assessment in order to keep pace with ever-increasing knowledge surrounding tissue-formation processes.
3D printing of bone tissue and niches
The field of tissue engineering has strived to mimic the cellular and extracellular bone matrix as a means of restoring bone tissue and improving its functions in vivo. The tissue microenvironment, including bone and cartilage tissue, is a complex 3D structure that provides a template for cell adhesion and initiates bone repair in vivo.80,81 Microenvironments also permit the regulation of nutrients and molecules and their transport to the innermost regions of the scaffolds to enable cell growth, vascularisation and waste material removal.81,82 Therefore, the properties of biomaterials including material and cellular composition, pore size, volume and mechanical strength are vital parameters that define their performance. Conventional fabrication techniques such as chemical/gas foaming,83 particle/salt leaching84 and thermally induced phase separation85 lack the ability to generate the complex 3D structure and material properties needed to replicate biological tissues. In an attempt to achieve better adaptability, the field has utilised additive manufacturing methods such as 3D printing. The feasibility of 3D printing as a means for generating biomimetic scaffolds for fracture repair was demonstrated by Inzana and colleagues who optimised a process for 3D printing of collagen/calcium phosphate.86 To assess the bone healing performance of the scaffolds, they were implanted into a critically sized murine femoral defect for a duration of 9 weeks. The implants displayed new bone formation, which incorporated the degrading scaffold material.
Another key step in producing biomimetic grafts is the ability to incorporate a cellular component into fabricated 3D biomaterials. Advances in biopolymer printing technology and materials has allowed for the fabrication of 3D constructs using alginate in combination with chondrocytes or human adipose-derived stem cells aimed towards cartilage tissue regeneration87,88 and osteochondral tissue fabrication.89 In particular, cartilage tissue engineering using additive 3D printing has gained momentum due to the limited ability of cells to incorporate into the dense avascular structure of cartilage. This could potentially be overcome through additive manufacturing, and further aided by the incorporation of key chondrogenic factors such as members of the TGF-β superfamily.90
Biomimetics through the use of natural target-tissue ECM
The concept of tissue engineering initially focused on developing materials that mimicked mature tissue, with the aim of incorporation into the host and subsequent remodelling, as defined by Langer and Vacanti in 1993.91 Initial attempts were made through the use of biodegradable scaffolds in combination with adult cells. However, in the context of bone repair, this approach has to date provided no clinically approved therapies. Due to this, current research towards improving the bioactive properties of regenerative implants through the use of naturally occurring target-tissue ECM has emerged. Indeed, the process of xenogeneic tissue decellularisation and its use for tissue engineering strategies within the field of regenerative medicine has been intensively studied.92–95
The process of decellularisation aims to remove all immunological components while leaving behind the ECM of the tissue and its associated growth factors, with the intention of maintaining the ECM proteins’ complex spatial arrangement.96 The ECM plays a key role in maintaining cell–matrix interactions that favour native tissue organisation and remodelling.38 Importantly, the structural and functional proteins in the ECM are well conserved within species. This high level of homology allows these matrices to be implanted in recipients of other species without rejection.97 The past decade has driven research towards novel biomaterials through the process of organ and tissue decellularisation. Some of the many examples of positive clinical results include the use of FDA-approved decellularised matrices such as porcine heart valves (Synergraft®; Cryolife) and acellular dermis (Alloderm®; LifeCell).98
The use of decellularised cartilage has drawn much attention due to its ability to harbour a large quantity of bioactive cues for tissue formation. The interaction of decellularised cartilage with resident cells, several chemotactic stimuli and activation of chondroinductive signalling pathways could result in continuous remodelling of the tissue. Decellularisation of cartilage, however, requires a vigorous protocol due to its dense nature. This is known to reduce the glycosaminoglycan (GAG) content and elasticity of the matrix.96 Despite this, the use of decellularised and lyophilised cartilage scaffolds has previously demonstrated bone formation in a rabbit model by Gawlitta and colleagues.99 The study involved coupling pre-primed chondrogenic MSCs with decellularised cartilage scaffolds, and demonstrated effective bone mineralisation when compared to the unseeded decellularised matrix. However, the contributing factors to the endochondral bone formation were unidentified, but could include components within the decellularised cartilaginous matrix, or factors produced by the cells as a result of the cell–matrix interaction.99 The choice of articular cartilage-derived scaffolds used in the study by Gawlitta et al. do pose limitations due to both tissue physiology and in vivo function. Articular surfaces are formed during pre-natal skeletal development and are highly stable during adult life.100 Indeed, factors such as chondromodulin 1 (ChM-I) have been implicated in the stability of articular cartilage by inhibiting EO in porcine models,101 and it has been further suggested that ChM-I functions as an inhibitor of angiogenesis, a process essential to endochondral remodelling.102,103 Additionally, a plethora of Wnt and BMP signalling modulators have been implicated in articular cartilage stability. Therefore, there is a requirement for a source of chondrogenic ECM-derived scaffolds that is intrinsically primed for endochondral remodelling. Other cartilaginous regions such as costal cartilage provide a promising option, as it gradually undergoes EO well into adult development.104 Furthermore, a study by Okihana and Shimomura105 indicated that when devitalised costal cartilage was implanted subcutaneously into rabbit and mouse models it underwent endochondral remodelling.
In summary, innovative decellularisation approaches may allow for the development of methodologies that minimise ECM damage. This, in combination with underexplored and more targeted tissue sources, may be the key to developing viable grafts that are able to mimic the endochondral repair process.
Stem cells for the creation of bone-forming tissue intermediates
Many of the key developments within the field of tissue engineering centre on the ability of cells to interact favourably with its carrier and mediate tissue formation and integration. It is therefore essential to investigate the interactions of key cell types in any regenerative approach in order to develop effective stem cell–based skeletal regenerative strategies.
Embryonic stem cells
The embryonic stem (ES) cell is derived from an early mammalian embryo and possesses a remarkable potential for differentiation into cell types from all three germ layers as demonstrated by Kaufman and colleagues using mouse embryos, termed ES cells.106 In vitro culture protocols paved the way for isolation and culture of the first human ES cell in 1998,107 where it was demonstrated that human ES cells could be kept in culture for up to 5 months followed by subsequent differentiation into all three embryonic germ layers. This facilitated further research into ES cell culture and differentiation programmes. Since then, ES cell research has generated promising results towards the treatment of diabetes,108 cardiovascular disease109–111 and musculoskeletal regeneration.112 In vitro differentiation of murine and human ES cells towards the osteogenic lineage has also been successfully achieved.113,114 Although multiple studies have shown that ESCs seeded onto scaffolds and primed in osteogenic media do not produce bone in vivo,115,116 the formation of bone within teratomas aligned with hypertrophic cartilage regions has been observed, indicating the capacity of ES cells to form bone through the developmental process of EO. This potential to form endochondral bone was confirmed with murine ESCs which were seeded onto ceramic scaffolds and differentiated towards a chondrogenic lineage using TGF-β.115 Furthermore, when implanted in vivo at an ectopic site into nude mice for 21 days, bone formation was observed on every one of the implanted cartilage tissue-engineered constructs (CTECs). Their capacity to form bone was further demonstrated when the CTECs were implanted orthotopically in rats with critical-size cranial defects. Similar results have also been obtained using human ESCs,117 thus highlighting the ability of ESCs to form bone via the endochondral pathway, hence mimicking both developmental skeletogenesis and fracture repair. Despite this huge potential of ESCs within tissue engineering and especially within the field of bone tissue engineering, their application is restricted by complex culture conditions, ethical constraints related to ESC isolation and their inherent tumour forming capacity.118,119
Induced pluripotent stem cells
There was a renewed interest in pluripotent stem cells when Takhashi and Yamanaka broke new ground by re-establishing the principles of developmental biology, which state that somatic cell differentiation is an irreversible process. Transfecting murine and human fibroblasts with the embryonic factors Oct4, c-Myc, Sox2 and Klf4 caused the regression of cell characteristics to a pluripotent, embryonic-like state, leading to them being named induced pluripotent stem cells (iPSCs).120,121 It was also demonstrated that like ES cells, iPSCs were able to form tissue from all three germ layers; however, iPSCs also had the inherent capacity to form teratomas (tumours) in vivo. Importantly, establishing a route for producing stem cell populations from a patient’s own cells overcomes many of the ethical issues faced by ES cell use.
In the context of bone repair, deriving progenitors with bone-forming potential from iPSCs has been an intensively studied area. Recent work has described a xeno-free-defined culture condition for the differentiation of iPSCs into iPSC-derived mesenchymal stem cells (iPS-MSCs), which were able to differentiate into chondrogenic, osteogenic and adipogenic lineages in vitro.122 Furthermore, when these cells were osteogenically differentiated for a period of 4 days and implanted into calvarial defects in immunocompromised mice, de novo bone formation originating from the implanted iPS-MSCs was observed.122 A recent study by Shey and colleagues has also demonstrated the efficacy of iPSC-MSCs for the treatment of non-union defects in mice.123 Furthermore, their chondrogenic differentiation capacity, and therefore their potential application towards endochondral tissue formation, has been demonstrated.124 The ability to create iPSC-derived cartilage constructs was also demonstrated with optimised culture conditions utilising scaffold-free hyaline cartilage tissue that displayed good integration into surrounding cartilage tissue when implanted, while not forming tumour masses.125 Although this study was targeted towards the treatment of cartilage defects, it is envisaged that differential stimulation of these cartilage constructs may allow the generation of implants capable of endochondral bone formation. Indeed, iPSCs have been shown to undergo chondrocyte hypertrophy in a similar manner to ESCs.117 Furthermore, the possibility of direct cellular reprogramming towards chondroprogenitors, a process that takes many of the concepts used to derive iPSCs, has demonstrated the ability of engineered cells to undergo in vitro hypertrophic differentiation. Implants containing these cell populations can drive endochondral bone formation and remodelling post implantation in nude mice.126
Mesenchymal stem cells
One of the most desirable properties when choosing a cell type for bone tissue engineering is the ability to isolate tissue-specific regenerative cell populations. As previously discussed, ESCs provide a highly malleable cell source, and the development of iPSCs has further advanced the field of personalised medicine through the generation of pluripotent populations from somatic cell sources. However, despite these developments, the most popular cell type still employed for the development of skeletal regeneration strategies is the mesenchymal stem cell (MSC). MSCs are widely recognised for their ability to differentiate towards osteochondral lineages127,128 and can be derived from several tissue sources. The most commonly used source of MSCs is the bone marrow, from which cells are isolated through the extraction of a bone marrow aspirate.129 Another common source of MSCs is adipose tissue from which MSCs (perycites) are isolated from digested fat tissue.130
Caplan first coined the term MSC in 1991.131 It was, however, in the 1960s and 1970s that seminal studies by Friedenstein isolated mesenchymal stromal cells and revealed their osteogenic potential by heterotopic transplantation.132,133 Since then, MSCs have been defined by the minimal criteria of positive expression of CD105, CD73 and CD90 and negativity for CD45, CD34, CD14, CD79α and HLA-DR.134 MSCs have been extensively used and reviewed for their clinical applicability within the field of bone tissue engineering.135,136 In relation to the treatment of bone fracture repair, it has been shown that the delivery of allogenic BM-MSCs in combination with demineralised bone matrix enhances fracture healing in clinical models of diabetes mellitus in rats.137 Similar results are also demonstrated by several other studies incorporating MSCs with scaffolding material for the treatment of fractures, particularly in their proposed use in non-union fractures.138–140
Until now, the treatment of large bone defects has largely relied on approaches that aim to harness the intramembranous pathway of bone regeneration. However, as discussed previously, recapitulation of EO may be more efficacious. Martin and colleagues applied this approach by creating cartilage constructs in vitro using human BM-MSC pellet cultures. Hypertrophy within these engineered constructs was induced through the withdrawal of TFG-β and the introduction of β-glycerophosphate with thyroxin. The resulting constructs displayed increased collagen type X deposition, typical of hypertrophic cartilage. Upon implantation in immunocompromised mice, these engineered hypertrophic cartilage constructs formed bone around the periphery at 4 weeks, with extensive endochondral bone formation after 8 weeks.7 More recent work has also illustrated that the addition of anti-inflammatory/tissue repair macrophages may further enhance the cartilage-forming capacity of BM-MSCs.141 This highlights the potential role of inflammatory cells during the fracture repair process; however, further orthotropic investigations are required to establish their clinical significance.
The periosteum – mimicking the master regulator of fracture repair
Despite the immense progress directed towards the design of fracture repair implants, the integration of all vital tissue properties and functions into a single system remains a major research challenge. With this in mind, the ability to mimic cell–matrix interactions with novel biomaterials, developed using natural matrices or engineered through systematic screening of polymers and surface topography of key relevance. However, if these systems are to be combined with cells, they should also be carefully selected to represent those modulating the fracture repair process.
The periosteum is a highly vascularised connective tissue that envelops the bone surface of long bones.142 It serves as a biophysical barrier to modulate the environmental conditions on the bone surface. The periosteum is composed of two distinct layers: an outer fibrous layer composed of fibroblastic cells in a collagen and elastin matrix, and an inner cambium layer, which provides a niche for a range of cell types, including fibroblasts and stem/progenitor cells.142,143 Recent prominent evidence has documented the regenerative potential of periosteal tissues and the functional capacity of periosteum-derived cells (PDCs) in the bone-healing process.144,145 The periosteum is predominantly responsible for 90% of cartilage and woven bone formation in the early fracture callus, with its removal significantly attenuating bone repair.143,146 In this respect, the periosteum has drawn great attention in pre-clinical bone tissue engineering approaches.11 The regenerative potential of the cells contained within the periosteum has been further demonstrated in vivo where they play a role in direct bone formation, as well as in chondrogenesis and EO.147
Consequently, great efforts have been made to target and isolate PDCs as a cell source for bone tissue engineering purposes. Previous work has shown that once these cells are inoculated into nude mice in scaffold144 and scaffold-free systems,147 they give rise to bone and cartilage tissue. Indeed, murine PDCs expanded in the presence of FGF demonstrated an enhanced capacity for in vivo bone production mediated by BMP-2 via the endochondral pathway, a characteristic unique to PDCs. Although culture-expanded PDCs have increased our knowledge and understanding of the periosteum, in vivo targeting of these cells within their niche, or replication of this tissue and therefore mimicking the role of the periosteum are both attractive avenues for further investigation.
Indeed, the fracture-healing process can be enhanced by mimicking periosteum–bone interactions. We have previously shown that human PDCs (hPDCs) that are seeded onto natural decellularised/devitalised bone matrix have the ability to undergo endochondral bone formation resulting in the formation of a bone organ containing a haematopoietic compartment.144 This process is driven by early PKC, BMP and Wnt signalling148 and can be further enhanced through the expansion of periosteal stem cells in humanised conditions.149 This inherently shows that the closer that the biological systems are mimicked, the more efficacious the system. Further work on this cell population has displayed the importance of mitogenic growth factors such as BMPs150 and cell–cell interactions151 as key regulators of the in vivo response to periosteal cell implants. Interestingly, combinatorial screening of growth factors involved in skeletogenesis reveals specific conditions that can direct PDCs towards stable cartilage152 and biphasic tissues with the potential capacity for osteochondral repair.
The fracture-healing site is known to be intrinsically responsive to key growth factors such as BMP, β-catenin/wingless-related factors (Wnt), TGFβ1 and FGFs which are released by cells to recruit and trigger hPDC in the periosteum. Further to this, we have reported the importance of BMP, β-catenin/Wnt, cAMP Response Element-Binding Protein (CREB), TGFβ, Endothelial Growth Factor (EGF) and Extracellular Signal-Regulated Kinase (Erk) signalling to bone formation from periosteal cells.153 Integrating the bioactive cues found within the niche of periosteal progenitor cells has been proposed as a vital step in developing a successful transplantable scaffold that promotes bone regeneration. Indeed, this may be achieved through the use of native decellularised matrix, for which proof of principle confirming the biocompatibility of decellularised periosteum has been delivered.154
Outlook and perspectives
As a means of repairing critical-sized bone defects, tissue engineering aims to recapitulate the biological processes involved with the formation of the various tissues that modulate the fracture-healing process. The development of scaffolds that possess the necessary cell-interaction properties and biological cues to ensure cellular survival, proliferation and differentiation of either native or infiltrating cells is progressing. Multiple efforts have succeeded with a plethora of studies yet to follow; however, a number of limitations have hindered the field of fracture repair tissue engineering. These include ensuring the engineered scaffolds are able to maintain their functional characteristics post implantation for the duration of the remodelling process,155 and lack of suitable biomaterials and characterised cells. The mechanical properties of the scaffold are critical to the regulation of mechano-transduction and cellular behaviour, affecting the cells’ potential to differentiate, develop and regenerate components that are key to the bone remodelling process.156 Many tissue-engineered constructs have demonstrated potential regenerative capabilities in vitro; however, upon implantation into in vivo models, this regenerative capacity can be lost, predominantly as a result of insufficient vascularisation and integration/remodelling of the scaffold with recipient tissue. Previous studies have therefore suggested allowing cells to form a microvasculature in the scaffold prior to implantation to improve vascularisation.157 Furthermore, the production of a biomimetic scaffold or niche may be subjective due to the age-related bone repair processes that occur in vivo. Implanting a model within a young individual may result in a rapid healing, yet the same scaffold may demonstrate the contrary in elderly patients. An aspect quite often neglected is the maintenance of the cell characteristics to provide a pool of progenitors capable of directing the full cascade of tissue repair. The use of stem cells in bone implants is a promising approach; however, knowledge gaps related to isolation, expansion, differentiation and tissue integration are likely to limit short- to mid-term clinical translation. It is proposed herein that the creation of a tissue module that can stimulate the environment prior to implantation, the formation of a material that can attract endogenous cells through specific cell-interaction motifs, or the in vivo targeting of stem cell niches are promising solutions for cases of delayed or non-union bone fractures.
Acknowledgments
Shared senior authorship: Helen C. Owen and Scott J. Roberts.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Orthopaedic Research UK (ORUK).
References
- 1. Marsell R, Einhorn TA. The biology of fracture healing. Injury 2011; 42(6): 551–555. DOI: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Einhorn TA. The science of fracture healing. J Orthop Trauma 2005; 19(Suppl. 10): S4–S6. [DOI] [PubMed] [Google Scholar]
- 3. Bishop JA, Palanca AA, Bellino MJ, et al. Assessment of compromised fracture healing. J Am Acad Orthop Surg 2012; 20(5): 273–282, http://www.ncbi.nlm.nih.gov/pubmed/22553099 [DOI] [PubMed] [Google Scholar]
- 4. Buza JA, III, Einhorn T. Bone healing in 2016. Clin Cases Miner Bone Metab 2016; 13(2): 101–105, http://www.ncbi.nlm.nih.gov/pubmed/27920804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mills LA, Simpson AH. The relative incidence of fracture non-union in the Scottish population (5.17 million): a 5-year epidemiological study. BMJ Open 2013; 3(2): e002276, http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3586107&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marsell R, Einhorn TA. Emerging bone healing therapies. J Orthop Trauma 2010; 24(3): S4–S8. [DOI] [PubMed] [Google Scholar]
- 7. Scotti C, Tonnarelli B, Papadimitropoulos A, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci U S A 2010; 107(16): 7251–7256, http://www.ncbi.nlm.nih.gov/pubmed/20406908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farrell E, van der Jagt OP, Koevoet W, et al. Chondrogenic priming of human bone marrow stromal cells: a better route to bone repair? Tissue Eng Part C Methods 2009; 15(2): 285–295. [DOI] [PubMed] [Google Scholar]
- 9. Tortelli F, Tasso R, Loiacono F, et al. The development of tissue-engineered bone of different origin through endochondral and intramembranous ossification following the implantation of mesenchymal stem cells and osteoblasts in a murine model. Biomaterials 2010; 31(2): 242–249, http://www.ncbi.nlm.nih.gov/pubmed/19796807 [DOI] [PubMed] [Google Scholar]
- 10. Dennis SC, Berkland CJ, Bonewald LF, et al. Endochondral ossification for enhancing bone regeneration: converging native extracellular matrix biomaterials and developmental engineering in vivo. Tissue Eng Part B Rev 2015; 21(3): 247–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roberts SJ, van Gastel N, Carmeliet G, et al. Uncovering the periosteum for skeletal regeneration: the stem cell that lies beneath. Bone 2015; 70: 10–18, http://www.ncbi.nlm.nih.gov/pubmed/25193160 [DOI] [PubMed] [Google Scholar]
- 12. Lange J, Sapozhnikova A, Lu C, et al. Action of IL-1beta during fracture healing. J Orthop Res 2010; 28(6): 778–784, http://www.ncbi.nlm.nih.gov/pubmed/20041490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al-Sebaei MO, Daukss DM, Belkina AC, et al. Role of Fas and Treg cells in fracture healing as characterized in the fas-deficient (lpr) mouse model of lupus. J Bone Miner Res 2014; 29(6): 1478–1491, http://www.ncbi.nlm.nih.gov/pubmed/24677136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang X, Ricciardi BF, Hernandez-Soria A, et al. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone 2007; 41(6): 928–936, http://www.ncbi.nlm.nih.gov/pubmed/17921078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wallace A, Cooney TE, Englund R, et al. Effects of interleukin-6 ablation on fracture healing in mice. J Orthop Res 2011; 29(9): 1437–1442. [DOI] [PubMed] [Google Scholar]
- 16. Gerstenfeld LC, Cho TJ, Kon T, et al. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res 2003; 18(9): 1584–1592, http://www.ncbi.nlm.nih.gov/pubmed/12968667 [DOI] [PubMed] [Google Scholar]
- 17. Glass GE, Chan JK, Freidin A, et al. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A 2011; 108(4): 1585–1590, http://www.ncbi.nlm.nih.gov/pubmed/21209334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nam D, Mau E, Wang Y, et al. T-lymphocytes enable osteoblast maturation via IL-17F during the early phase of fracture repair. PLoS ONE 2012; 7(6): e40044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ono T, Okamoto K, Nakashima T, et al. IL-17-producing γδ T cells enhance bone regeneration. Nat Commun 2016; 7: 10928, http://www.ncbi.nlm.nih.gov/pubmed/26965320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han X, Yang Q, Lin L, et al. Interleukin-17 enhances immunosuppression by mesenchymal stem cells. Cell Death Differ 2014; 21(11): 1758–1768, http://www.ncbi.nlm.nih.gov/pubmed/25034782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Toben D, Schroeder I, El Khassawna T, et al. Fracture healing is accelerated in the absence of the adaptive immune system. J Bone Miner Res 2011; 26(1): 113–124. [DOI] [PubMed] [Google Scholar]
- 22. Gerstenfeld LC, Cullinane DM, Barnes GL, et al. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem 2003; 88(5): 873–884. [DOI] [PubMed] [Google Scholar]
- 23. Colburn NT, Zaal KJ, Wang F, et al. A role for gamma/delta T cells in a mouse model of fracture healing. Arthritis Rheum 2009; 60(6): 1694–1703, http://www.ncbi.nlm.nih.gov/pubmed/19479830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maes C, Carmeliet G, Schipani E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat Rev Rheumatol 2012; 8(6): 358–366, http://www.ncbi.nlm.nih.gov/pubmed/22450551 [DOI] [PubMed] [Google Scholar]
- 25. Cho T-J, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res 2002; 17(3): 513–520, http://www.ncbi.nlm.nih.gov/pubmed/11874242 [DOI] [PubMed] [Google Scholar]
- 26. Schmid GJ, Kobayashi C, Sandell LJ, et al. Fibroblast growth factor expression during skeletal fracture healing in mice. Dev Dyn 2009; 238(3): 766–774, http://www.ncbi.nlm.nih.gov/pubmed/19235733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu YY, Lieu S, Lu C, et al. Immunolocalization of BMPs, BMP antagonists, receptors, and effectors during fracture repair. Bone 2010; 46(3): 841–851, http://www.ncbi.nlm.nih.gov/pubmed/19913648 [DOI] [PubMed] [Google Scholar]
- 28. AI-Aql ZS, Alagl AS, Graves DT, et al. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res 2008; 87(2): 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thompson Z, Miclau T, Hu D, et al. A model for intramembranous ossification during fracture healing. J Orthop Res 2002; 20(5): 1091–1098. [DOI] [PubMed] [Google Scholar]
- 30. Santolini E, West R, Giannoudis PV. Risk factors for long bone fracture non-union: a stratification approach based on the level of the existing scientific evidence. Injury 2015; 46(Suppl. 8): S8–S19, http://www.ncbi.nlm.nih.gov/pubmed/26747924 [DOI] [PubMed] [Google Scholar]
- 31. Graves DT, Alblowi J, Paglia DN, et al. Impact of diabetes on fracture healing. J Exp Clin Med 2011; 3(1): 3–8. [Google Scholar]
- 32. Castillo RC, Bosse MJ, MacKenzie EJ, et al. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma 2005; 19(3): 151–157. [DOI] [PubMed] [Google Scholar]
- 33. Giannoudis P, Tzioupis C, Almalki T, et al. Fracture healing in osteoporotic fractures: is it really different? a basic science perspective. Injury 2007; 38(Suppl. 1): S90–S99. [DOI] [PubMed] [Google Scholar]
- 34. Panteli M, Pountos I, Jones E, et al. Biological and molecular profile of fracture non-union tissue: current insights. J Cell Mol Med 2015; 19(4): 685–713, http://www.ncbi.nlm.nih.gov/pubmed/25726940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lenas P, Moos M, Luyten FP. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part I: from three-dimensional cell growth to biomimetics of in vivo development. Tissue Eng Part B Rev 2009; 15(4): 381–394, http://www.ncbi.nlm.nih.gov/pubmed/19505199 [DOI] [PubMed] [Google Scholar]
- 36. Lenas P, Moos M, Luyten FP. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part II: from genes to networks: tissue engineering from the viewpoint of systems biology and network science. Tissue Eng Part B Rev 2009; 15(4): 395–422, http://www.ncbi.nlm.nih.gov/pubmed/19589040 [DOI] [PubMed] [Google Scholar]
- 37. Hadjiargyrou M, O’Keefe RJ. The convergence of fracture repair and stem cells: interplay of genes, aging, environmental factors and disease. J Bone Miner Res 2014; 29(11): 2307–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta 2014; 1840(8): 2506–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Theocharis AD, Skandalis SS, Gialeli C, et al. Extracellular matrix structure. Adv Drug Deliv Rev 2016; 97: 4–27, http://www.ncbi.nlm.nih.gov/pubmed/26562801 [DOI] [PubMed] [Google Scholar]
- 40. Gentili C, Cancedda R. Cartilage and bone extracellular matrix. Curr Pharm Des 2009; 15(12): 1334–1348. [DOI] [PubMed] [Google Scholar]
- 41. Yue B. Biology of the extracellular matrix: an overview. J Glaucoma 23(8): S20–S23, http://www.ncbi.nlm.nih.gov/pubmed/25275899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim S-H, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol 2011; 209(2): 139–151, http://www.ncbi.nlm.nih.gov/pubmed/21307119 [DOI] [PubMed] [Google Scholar]
- 43. Pişkin E. Biodegradable polymers as biomaterials. J Biomater Sci Polym Ed 1995; 6(9): 775–795, http://www.ncbi.nlm.nih.gov/pubmed/7772566 [DOI] [PubMed] [Google Scholar]
- 44. Ji Y, Ghosh K, Shu XZ, et al. Electrospun three-dimensional hyaluronic acid nanofibrous scaffolds. Biomaterials 2006; 27(20): 3782–3792, http://www.ncbi.nlm.nih.gov/pubmed/16556462 [DOI] [PubMed] [Google Scholar]
- 45. Tabata Y. Biomaterial technology for tissue engineering applications. J R Soc Interface 2009; 6(Suppl. 3): S311–S324, http://www.ncbi.nlm.nih.gov/pubmed/19324684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alexander H, Brunski J, Cooper S. Classes of materials used in medicine. In: BD Ratner, AS Hoffman, FJ Schoen. (eds) Biomaterials science: an introduction to materials in medicine, 1996, https://books.google.com/books?hl=en&lr=&id=pZFzd8GtUe8C&oi=fnd&pg=PA37&ots=J8aYUxkqF1&sig=RfVi4itRhRqT33VuA70uaMFwJWQ
- 47. He C, Xiao G, Jin X, et al. Electrodeposition on nanofibrous polymer scaffolds: rapid mineralization, tunable calcium phosphate composition and topography. Adv Funct Mater 2010; 20(20): 3568–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu C, Ramaswamy Y, Zhu Y, et al. The effect of mesoporous bioactive glass on the physiochemical, biological and drug-release properties of poly(DL-lactide-co-glycolide) films. Biomaterials 2009; 30(12): 2199–2208. [DOI] [PubMed] [Google Scholar]
- 49. Orefice R, Clark A, West J, et al. Processing, properties, and in vitro bioactivity of polysulfone-bioactive glass composites. J Biomed Mater Res A 2007; 80(3): 565–580, http://www.ncbi.nlm.nih.gov/pubmed/17031819 [DOI] [PubMed] [Google Scholar]
- 50. Zhang P, Hong Z, Yu T, et al. In vivo mineralization and osteogenesis of nanocomposite scaffold of poly(lactide-co-glycolide) and hydroxyapatite surface-grafted with poly(l-lactide). Biomaterials 2009; 30(1): 58–70. [DOI] [PubMed] [Google Scholar]
- 51. Ulery BD, Nair LS, Laurencin CT. Biomedical applications of biodegradable polymers. J Polym Sci B Polym Phys 2011; 49(12): 832–864, http://www.ncbi.nlm.nih.gov/pubmed/21769165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Girones Molera J, Mendez JA, San Roman J. Bioresorbable and nonresorbable polymers for bone tissue engineering. Curr Pharm Des 2012; 18(18): 2536–2557, http://www.ncbi.nlm.nih.gov/pubmed/22512444 [DOI] [PubMed] [Google Scholar]
- 53. Guo X, Wang C, Duan C, et al. Repair of osteochondral defects with autologous chondrocytes seeded onto bioceramic scaffold in sheep. Tissue Eng 10(11–12): 1830–1840, http://www.ncbi.nlm.nih.gov/pubmed/15684691 [DOI] [PubMed] [Google Scholar]
- 54. Zignani M, Le Minh T, Einmahl S, et al. Improved biocompatibility of a viscous bioerodible poly(ortho ester) by controlling the environmental pH during degradation. Biomaterials 2000; 21(17): 1773–1778. [DOI] [PubMed] [Google Scholar]
- 55. Nooeaid P, Salih V, Beier JP, et al. Osteochondral tissue engineering: scaffolds, stem cells and applications. J Cell Mol Med 2012; 16(10): 2247–2270, http://www.ncbi.nlm.nih.gov/pubmed/22452848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shen H, Hu X, Yang F, et al. Cell affinity for bFGF immobilized heparin-containing poly(lactide-co-glycolide) scaffolds. Biomaterials 2011; 32(13): 3404–3412. [DOI] [PubMed] [Google Scholar]
- 57. Kantawong F, Burgess KE, Jayawardena K, et al. Whole proteome analysis of osteoprogenitor differentiation induced by disordered nanotopography and mediated by ERK signalling. Biomaterials 2009; 30(27): 4723–4731, http://www.ncbi.nlm.nih.gov/pubmed/19560200 [DOI] [PubMed] [Google Scholar]
- 58. Biggs MJ, Richards RG, Gadegaard N, et al. The use of nanoscale topography to modulate the dynamics of adhesion formation in primary osteoblasts and ERK/MAPK signalling in STRO-1+ enriched skeletal stem cells. Biomaterials 2009; 30(28): 5094–5103, http://www.ncbi.nlm.nih.gov/pubmed/19539986 [DOI] [PubMed] [Google Scholar]
- 59. Biggs MJ, Richards RG, Gadegaard N, et al. Interactions with nanoscale topography: adhesion quantification and signal transduction in cells of osteogenic and multipotent lineage. J Biomed Mater Res A 2009; 91(1): 195–208, http://www.ncbi.nlm.nih.gov/pubmed/18814275 [DOI] [PubMed] [Google Scholar]
- 60. Shafiee A, Seyedjafari E, Sadat Taherzadeh E, et al. Enhanced chondrogenesis of human nasal septum derived progenitors on nanofibrous scaffolds. Mater Sci Eng C 2014; 40: 445–454. [DOI] [PubMed] [Google Scholar]
- 61. Wu Y, Yang Z, Law JB, et al. The combined effect of substrate stiffness and surface topography on chondrogenic differentiation of mesenchymal stem cells. Tissue Eng Part A 2017; 23(1–2): 43–54, http://www.ncbi.nlm.nih.gov/pubmed/27824280 [DOI] [PubMed] [Google Scholar]
- 62. Baker BM, Nathan AS, Gee AO, et al. The influence of an aligned nanofibrous topography on human mesenchymal stem cell fibrochondrogenesis. Biomaterials 2010; 31(24): 6190–6200, http://www.ncbi.nlm.nih.gov/pubmed/20494438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yin Z, Chen X, Song H-X, et al. Electrospun scaffolds for multiple tissues regeneration in vivo through topography dependent induction of lineage specific differentiation. Biomaterials 2015; 44: 173–185, http://www.ncbi.nlm.nih.gov/pubmed/25617136 [DOI] [PubMed] [Google Scholar]
- 64. Neves SC, Mota C, Longoni A, et al. Additive manufactured polymeric 3D scaffolds with tailored surface topography influence mesenchymal stromal cells activity. Biofabrication 2016; 8(2): 025012, http://www.ncbi.nlm.nih.gov/pubmed/27219645 [DOI] [PubMed] [Google Scholar]
- 65. Balasundaram G, Storey DM, Webster TJ. Novel nano-rough polymers for cartilage tissue engineering. Int J Nanomedicine 2014; 9: 1845–1853, http://www.ncbi.nlm.nih.gov/pubmed/24790427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ranella A, Barberoglou M, Bakogianni S, et al. Tuning cell adhesion by controlling the roughness and wettability of 3D micro/nano silicon structures. Acta Biomater 2010; 6(7): 2711–2720. [DOI] [PubMed] [Google Scholar]
- 67. Benoit DS, Schwartz MP, Durney AR, et al. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater 2008; 7(10): 816–823, http://www.ncbi.nlm.nih.gov/pubmed/18724374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mei Y, Elliott JT, Smith JR, et al. Gradient substrate assembly for quantifying cellular response to biomaterials. J Biomed Mater Res A 2006; 79(4): 974–988, http://www.ncbi.nlm.nih.gov/pubmed/16948143 [DOI] [PubMed] [Google Scholar]
- 69. Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature 2009; 462(7272): 433–441, http://www.ncbi.nlm.nih.gov/pubmed/19940913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Coyle R, Jia J, Mei Y. Polymer microarray technology for stem cell engineering. Acta Biomater 2016; 34: 60–72, http://www.ncbi.nlm.nih.gov/pubmed/26497624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat Biotechnol 2004; 22(7): 863–866, http://www.ncbi.nlm.nih.gov/pubmed/15195101 [DOI] [PubMed] [Google Scholar]
- 72. Mei Y, Saha K, Bogatyrev SR, et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat Mater 2010; 9(9): 768–778, http://www.ncbi.nlm.nih.gov/pubmed/20729850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Anderson DG, Putnam D, Lavik EB, et al. Biomaterial microarrays: rapid, microscale screening of polymer-cell interaction. Biomaterials 2005; 26(23): 4892–4897, http://www.ncbi.nlm.nih.gov/pubmed/15763269 [DOI] [PubMed] [Google Scholar]
- 74. Wrighton PJ, Klim JR, Hernandez BA, et al. Signals from the surface modulate differentiation of human pluripotent stem cells through glycosaminoglycans and integrins. Proc Natl Acad Sci U S A 2014; 111(51): 18126–18131, http://www.ncbi.nlm.nih.gov/pubmed/25422477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tare RS, Khan F, Tourniaire G, et al. A microarray approach to the identification of polyurethanes for the isolation of human skeletal progenitor cells and augmentation of skeletal cell growth. Biomaterials 2009; 30(6): 1045–1055. [DOI] [PubMed] [Google Scholar]
- 76. Duffy CRE, Zhang R, How S-E, et al. A high-throughput polymer microarray approach for identifying defined substrates for mesenchymal stem cells. Biomater Sci 2014; 2(11): 1683–1692, http://xlink.rsc.org/?DOI=C4BM00112E [DOI] [PubMed] [Google Scholar]
- 77. Hulsman M, Hulshof F, Unadkat H, et al. Analysis of high-throughput screening reveals the effect of surface topographies on cellular morphology. Acta Biomater 2015; 15: 29–38. [DOI] [PubMed] [Google Scholar]
- 78. Unadkat HV, Hulsman M, Cornelissen K, et al. An algorithm-based topographical biomaterials library to instruct cell fate. Proc Natl Acad Sci U S A 2011; 108(40): 16565–16570, http://www.ncbi.nlm.nih.gov/pubmed/21949368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Unadkat HV, Rewagad RR, Hulsman M, et al. A modular versatile chip carrier for high-throughput screening of cell-biomaterial interactions. J R Soc Interface 2013; 10(78): 20120753, http://www.ncbi.nlm.nih.gov/pubmed/23152103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. O’Brien CM, Holmes B, Faucett S, et al. Three-dimensional printing of nanomaterial scaffolds for complex tissue regeneration. Tissue Eng Part B Rev 2015; 21(1): 103–114, http://www.ncbi.nlm.nih.gov/pubmed/25084122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today 2013; 16(12): 496–504. [Google Scholar]
- 82. Jones AC, Arns CH, Sheppard AP, et al. Assessment of bone ingrowth into porous biomaterials using MICRO-CT. Biomaterials 2007; 28(15): 2491–2504. [DOI] [PubMed] [Google Scholar]
- 83. Kucharska M, Butruk B, Walenko K, et al. Fabrication of in-situ foamed chitosan/β-TCP scaffolds for bone tissue engineering application. Mater Lett 2012; 85: 124–127. [Google Scholar]
- 84. Stoppato M, Carletti E, Sidarovich V, et al. Influence of scaffold pore size on collagen I development: a new in vitro evaluation perspective. J Bioact Compat Polym 2013; 28(1): 16–32. [Google Scholar]
- 85. Pavia FC, La Carrubba V, Piccarolo S, et al. Polymeric scaffolds prepared via thermally induced phase separation: tuning of structure and morphology. J Biomed Mater Res A 2008; 86(2): 459–466, http://www.ncbi.nlm.nih.gov/pubmed/17975822 [DOI] [PubMed] [Google Scholar]
- 86. Inzana JA, Olvera D, Fuller SM, et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014; 35(13): 4026–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lee J-S, Hong JM, Jung JW, et al. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication 2014; 6(2): 24103, http://www.ncbi.nlm.nih.gov/pubmed/24464765 [DOI] [PubMed] [Google Scholar]
- 88. Kundu J, Shim J-H, Jang J, et al. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J Tissue Eng Regen Med 2015; 9(11): 1286–1297, http://www.ncbi.nlm.nih.gov/pubmed/23349081 [DOI] [PubMed] [Google Scholar]
- 89. Fedorovich NE, Schuurman W, Wijnberg HM, et al. Biofabrication of osteochondral tissue equivalents by printing topologically defined, cell-laden hydrogel scaffolds. Tissue Eng Part C Methods 2012; 18(1): 33–44, http://www.ncbi.nlm.nih.gov/pubmed/21854293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Madry H, Rey-Rico A, Venkatesan JK, et al. Transforming growth factor Beta-releasing scaffolds for cartilage tissue engineering. Tissue Eng Part B Rev 2014; 20(2): 106–125, http://www.ncbi.nlm.nih.gov/pubmed/23815376 [DOI] [PubMed] [Google Scholar]
- 91. Langer R, Vacanti JP. Tissue engineering. Science 1993; 260: 920–926. [DOI] [PubMed] [Google Scholar]
- 92. Livesey SA, Herndon DN, Hollyoak MA, et al. Transplanted acellular allograft dermal matrix. Potential as a template for the reconstruction of viable dermis. Transplantation 1995; 60(1): 1–9. [PubMed] [Google Scholar]
- 93. Sutherland RS, Baskin LS, Hayward SW, et al. Regeneration of bladder urothelium, smooth muscle, blood vessels and nerves into an acellular tissue matrix. J Urol 1996; 156: 571–577. [DOI] [PubMed] [Google Scholar]
- 94. Mazza G, Rombouts K, Rennie Hall A, et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci Rep 2015; 5: 13079, http://www.nature.com/articles/srep13079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Guyette JP, Gilpin SE, Charest JM, et al. Perfusion decellularization of whole organs. Nat Protoc 2014; 9(6): 1451–1468, http://www.nature.com/doifinder/10.1038/nprot.2014.097 [DOI] [PubMed] [Google Scholar]
- 96. Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials 2011; 32(12): 3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials 2006; 27: 3675–3683. [DOI] [PubMed] [Google Scholar]
- 98. Cheng CW, Solorio LD, Alsberg E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol Adv 2014; 32: 462–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gawlitta D, Benders KEM, Visser J, et al. Decellularized cartilage-derived matrix as substrate for endochondral bone regeneration. Tissue Eng Part A 2015; 21(3–4): 694–703, http://online.liebertpub.com/doi/abs/10.1089/ten.tea.2014.0117 [DOI] [PubMed] [Google Scholar]
- 100. Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol 2000; 16: 191–220, http://www.ncbi.nlm.nih.gov/pubmed/11031235 [DOI] [PubMed] [Google Scholar]
- 101. Klinger P, Surmann-Schmitt C, Brem M, et al. Chondromodulin 1 stabilizes the chondrocyte phenotype and inhibits endochondral ossification of porcine cartilage repair tissue. Arthritis Rheum 2011; 63(9): 2721–2731. [DOI] [PubMed] [Google Scholar]
- 102. Kitahara H, Hayami T, Tokunaga K, et al. Chondromodulin-I expression in rat articular cartilage. Arch Histol Cytol 2003; 66(3): 221–228, http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14527163 [DOI] [PubMed] [Google Scholar]
- 103. Hayami T, Funaki H, Yaoeda K, et al. Expression of the cartilage derived anti-angiogenic factor chondromodulin-I decreases in the early stage of experimental osteoarthritis. J Rheumatol 2003; 30(10): 2207–2217. [PubMed] [Google Scholar]
- 104. Bahrami S, Plate U, Dreier R, et al. Endochondral ossification of costal cartilage is arrested after chondrocytes have reached hypertrophic stage of late differentiation. Matrix Biol 2001; 19(8): 707–715. [DOI] [PubMed] [Google Scholar]
- 105. Okihana H, Shimomura Y. Osteogenic activity of growth cartilage examined by implanting decalcified or devitalized ribs and costal cartilage zone, and living growth cartilage cells. Bone 1992; 13(5): 387–393. [DOI] [PubMed] [Google Scholar]
- 106. Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981; 292(5819): 154–156, http://www.ncbi.nlm.nih.gov/pubmed/7242681 [DOI] [PubMed] [Google Scholar]
- 107. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282(5391): 1145–1147, http://www.ncbi.nlm.nih.gov/pubmed/9804556 [DOI] [PubMed] [Google Scholar]
- 108. Godfrey KJ, Mathew B, Bulman JC, et al. Stem cell-based treatments for Type 1 diabetes mellitus: bone marrow, embryonic, hepatic, pancreatic and induced pluripotent stem cells. Diabet Med 2012; 29(1): 14–23, http://www.ncbi.nlm.nih.gov/pubmed/21883442 [DOI] [PubMed] [Google Scholar]
- 109. Shabani P, Ghazizadeh Z, Pahlavan S, et al. Exogenous treatment with eicosapentaenoic acid supports maturation of cardiomyocytes derived from embryonic stem cells. Biochem Biophys Res Commun 2015; 461(2): 281–286, http://www.ncbi.nlm.nih.gov/pubmed/25871791 [DOI] [PubMed] [Google Scholar]
- 110. Sekiguchi H, Ii M, Losordo DW. The relative potency and safety of endothelial progenitor cells and unselected mononuclear cells for recovery from myocardial infarction and ischemia. J Cell Physiol 2009; 219(2): 235–242, http://www.ncbi.nlm.nih.gov/pubmed/19115244 [DOI] [PubMed] [Google Scholar]
- 111. Song Y-H, Pinkernell K, Alt E. Stem cell induced cardiac regeneration: fusion/mitochondrial exchange and/or transdifferentiation? Cell Cycle 2011; 10(14): 2281–2286, http://www.ncbi.nlm.nih.gov/pubmed/21654195 [DOI] [PubMed] [Google Scholar]
- 112. Quattrocelli M, Cassano M, Crippa S, et al. Cell therapy strategies and improvements for muscular dystrophy. Cell Death Differ 2010; 17(8): 1222–1229, http://www.ncbi.nlm.nih.gov/pubmed/19876070 [DOI] [PubMed] [Google Scholar]
- 113. Buttery LD, Bourne S, Xynos JD, et al. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng 2001; 7(1): 89–99, http://www.ncbi.nlm.nih.gov/pubmed/11224927 [DOI] [PubMed] [Google Scholar]
- 114. Sottile V, Thomson A, McWhir J. In vitro osteogenic differentiation of human ES cells. Cloning Stem Cells 2003; 5(2): 149–155, http://www.ncbi.nlm.nih.gov/pubmed/12930627 [DOI] [PubMed] [Google Scholar]
- 115. Jukes JM, Both SK, Leusink A, et al. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci U S A 2008; 105(19): 6840–6845, http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2374550&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bielby RC, Boccaccini AR, Polak JM, et al. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng 10(9–10): 1518–1525, http://www.ncbi.nlm.nih.gov/pubmed/15588411 [DOI] [PubMed] [Google Scholar]
- 117. Craft AM, Rockel JS, Nartiss Y, et al. Generation of articular chondrocytes from human pluripotent stem cells. Nat Biotechnol 2015; 33(6): 638–645, http://www.ncbi.nlm.nih.gov/pubmed/25961409 [DOI] [PubMed] [Google Scholar]
- 118. King NM, Perrin J. Ethical issues in stem cell research and therapy. Stem Cell Res Ther 2014; 5(4): 85, http://www.ncbi.nlm.nih.gov/pubmed/25157428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Andrews PW. From teratocarcinomas to embryonic stem cells. Philos Trans R Soc Lond B Biol Sci 2002; 357(1420): 405–417, http://www.ncbi.nlm.nih.gov/pubmed/12028783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126(4): 663–676, http://www.ncbi.nlm.nih.gov/pubmed/16904174 [DOI] [PubMed] [Google Scholar]
- 121. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131(5): 861–872, http://www.ncbi.nlm.nih.gov/pubmed/18035408 [DOI] [PubMed] [Google Scholar]
- 122. Villa-Diaz LG, Brown SE, Liu Y, et al. Derivation of mesenchymal stem cells from human induced pluripotent stem cells cultured on synthetic substrates. Stem Cells 2012; 30(6): 1174–1181, http://www.ncbi.nlm.nih.gov/pubmed/22415987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sheyn D, Ben-David S, Shapiro G, et al. Human induced pluripotent stem cells differentiate into functional mesenchymal stem cells and repair bone defects. Stem Cells Transl Med 2016; 5(11): 1447–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ko J-Y, Kim K-I, Park S, et al. In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials 2014; 35(11): 3571–3581, http://www.ncbi.nlm.nih.gov/pubmed/24462354 [DOI] [PubMed] [Google Scholar]
- 125. Yamashita A, Morioka M, Yahara Y, et al. Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem Cell Reports 2015; 4(3): 404–418, http://www.ncbi.nlm.nih.gov/pubmed/25733017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Tam WL, O DF, Hiramatsu K, et al. Sox9 reprogrammed dermal fibroblasts undergo hypertrophic differentiation in vitro and trigger endochondral ossification in vivo. Cell Reprogram 2014; 16(1): 29–39, http://www.ncbi.nlm.nih.gov/pubmed/24459991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 2012; 40(5): 363–408, http://www.ncbi.nlm.nih.gov/pubmed/23339648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bernardo ME, Pagliara D, Locatelli F. Mesenchymal stromal cell therapy: a revolution in regenerative medicine? Bone Marrow Transplant 2012; 47(2): 164–171, http://www.ncbi.nlm.nih.gov/pubmed/21478914 [DOI] [PubMed] [Google Scholar]
- 129. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284(5411): 143–147, http://www.ncbi.nlm.nih.gov/pubmed/10102814 [DOI] [PubMed] [Google Scholar]
- 130. Kuhbier JW, Weyand B, Radtke C, et al. Isolation, characterization, differentiation, and application of adipose-derived stem cells. Adv Biochem Eng Biotechnol 2010; 123: 55–105. [DOI] [PubMed] [Google Scholar]
- 131. Caplan AI. Mesenchymal stem cells. J Orthop Res 1991; 9(5): 641–650, http://www.ncbi.nlm.nih.gov/pubmed/1870029 [DOI] [PubMed] [Google Scholar]
- 132. Tavassoli M, Crosby WH. Transplantation of marrow to extramedullary sites. Science 1968; 161(3836): 54–56, http://www.ncbi.nlm.nih.gov/pubmed/4871792 [DOI] [PubMed] [Google Scholar]
- 133. Friedenstein AJ. Precursor cells of mechanocytes. Int Rev Cytol 1976; 47: 327–359. [DOI] [PubMed] [Google Scholar]
- 134. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8(4): 315–317. [DOI] [PubMed] [Google Scholar]
- 135. Arvidson K, Abdallah BM, Applegate LA, et al. Bone regeneration and stem cells. J Cell Mol Med 2011; 15(4): 718–746, http://www.ncbi.nlm.nih.gov/pubmed/21129153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Yousefi A-M, James PF, Akbarzadeh R, et al. Prospect of stem cells in bone tissue engineering: a review. Stem Cells Int 2016; 2016: 6180487, http://www.ncbi.nlm.nih.gov/pubmed/26880976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Breitbart EA, Meade S, Azad V, et al. Mesenchymal stem cells accelerate bone allograft incorporation in the presence of diabetes mellitus. J Orthop Res 2010; 28(7): 942–949, http://www.ncbi.nlm.nih.gov/pubmed/20058266 [DOI] [PubMed] [Google Scholar]
- 138. Gómez-Barrena E, Rosset P, Lozano D, et al. Bone fracture healing: cell therapy in delayed unions and nonunions. Bone 2015; 70: 93–101. [DOI] [PubMed] [Google Scholar]
- 139. Kawate K, Yajima H, Ohgushi H, et al. Tissue-engineered approach for the treatment of steroid-induced osteonecrosis of the femoral head: transplantation of autologous mesenchymal stem cells cultured with beta-tricalcium phosphate ceramics and free vascularized fibula. Artif Organs 2006; 30(12): 960–962, http://www.ncbi.nlm.nih.gov/pubmed/17181837 [DOI] [PubMed] [Google Scholar]
- 140. Han D, Han N, Zhang P, et al. Local transplantation of osteogenic pre-differentiated autologous adipose-derived mesenchymal stem cells may accelerate non-union fracture healing with limited pro-metastatic potency. Int J Clin Exp Med 2015; 8(1): 1406–1410, http://www.ncbi.nlm.nih.gov/pubmed/25785146 [PMC free article] [PubMed] [Google Scholar]
- 141. Sesia SB, Duhr R, Medeiros da, Cunha C, et al. Anti-inflammatory/tissue repair macrophages enhance the cartilage-forming capacity of human bone marrow-derived mesenchymal stromal cells. J Cell Physiol 2015; 230(6): 1258–1269, http://www.ncbi.nlm.nih.gov/pubmed/25413299 [DOI] [PubMed] [Google Scholar]
- 142. Allen MR, Hock JM, Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone 2004; 35(5): 1003–1012, http://www.ncbi.nlm.nih.gov/pubmed/15542024 [DOI] [PubMed] [Google Scholar]
- 143. Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res 2009; 24(2): 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Roberts SJ, Geris L, Kerckhofs G, et al. The combined bone forming capacity of human periosteal derived cells and calcium phosphates. Biomaterials 2011; 32(19): 4393–4405. [DOI] [PubMed] [Google Scholar]
- 145. Xie C, Ming X, Wang Q, et al. COX-2 from the injury milieu is critical for the initiation of periosteal progenitor cell mediated bone healing. Bone 2008; 43(6): 1075–1083, http://www.ncbi.nlm.nih.gov/pubmed/18773980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhang X, Xie C, Lin ASP, et al. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J Bone Miner Res 2005; 20(12): 2124–2137, http://www.ncbi.nlm.nih.gov/pubmed/16294266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Van Gastel N, Stegen S, Stockmans I, et al. Expansion of murine periosteal progenitor cells with fibroblast growth factor 2 reveals an intrinsic endochondral ossification program mediated by bone morphogenetic protein 2. Stem Cells 2014; 32(9): 2407–2418. [DOI] [PubMed] [Google Scholar]
- 148. Bolander J, Chai YC, Geris L, et al. Early BMP, Wnt and Ca(2+)/PKC pathway activation predicts the bone forming capacity of periosteal cells in combination with calcium phosphates. Biomaterials 2016; 86: 106–118, http://www.ncbi.nlm.nih.gov/pubmed/26901484 [DOI] [PubMed] [Google Scholar]
- 149. Roberts SJ, Owen HC, Tam WL, et al. Humanized culture of periosteal progenitors in allogeneic serum enhances osteogenic differentiation and in vivo bone formation. Stem Cells Transl Med 2014; 3(2): 218–228, http://www.ncbi.nlm.nih.gov/pubmed/24375540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Bolander J, Ji W, Geris L, et al. The combined mechanism of bone morphogenetic protein- and calcium phosphate-induced skeletal tissue formation by human periosteum derived cells. Eur Cell Mater 2016; 31: 11–25, http://www.ncbi.nlm.nih.gov/pubmed/26728496 [DOI] [PubMed] [Google Scholar]
- 151. Leijten J, Teixeira LSM, Bolander J, et al. Bioinspired seeding of biomaterials using three dimensional microtissues induces chondrogenic stem cell differentiation and cartilage formation under growth factor free conditions. Sci Rep 2016; 6: 36011, http://www.ncbi.nlm.nih.gov/pubmed/27808102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Mendes LF, Tam WL, Chai YC, et al. Combinatorial analysis of growth factors reveals the contribution of bone morphogenetic proteins to chondrogenic differentiation of human periosteal cells. Tissue Eng Part C Methods 2016; 22(5): 473–486, http://www.ncbi.nlm.nih.gov/pubmed/27018617 [DOI] [PubMed] [Google Scholar]
- 153. Eyckmans J, Roberts SJ, Bolander J, et al. Mapping calcium phosphate activated gene networks as a strategy for targeted osteoinduction of human progenitors. Biomaterials 2013; 34(19): 4612–4621, http://www.ncbi.nlm.nih.gov/pubmed/23537666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Chen K, Lin X, Zhang Q, et al. Decellularized periosteum as a potential biologic scaffold for bone tissue engineering. Acta Biomater 2015; 19: 46–55. [DOI] [PubMed] [Google Scholar]
- 155. Alaribe FN, Manoto SL, Motaung SCKM. Scaffolds from biomaterials: advantages and limitations in bone and tissue engineering. Biol 2016; 71(4): 353–366. [Google Scholar]
- 156. Thein-Han WW, Misra RDK. Biomimetic chitosan-nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater 2009; 5(4): 1182–1197. [DOI] [PubMed] [Google Scholar]
- 157. Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science 2009; 324(5935): 1673–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]