Abstract
Relaxin‐3 has been proposed to modulate emotional–behavioural functions such as arousal and behavioural activation, appetite regulation, stress responses, anxiety, memory, sleep and circadian rhythm. The nucleus incertus (NI), in the midline tegmentum close to the fourth ventricle, projects widely throughout the brain and is the primary site of relaxin‐3 neurons. Over recent years, a number of preclinical studies have explored the function of the NI and relaxin‐3 signalling, including reports of mRNA or peptide expression changes in the NI in response to behavioural or pharmacological manipulations, effects of lesions or electrical or pharmacological manipulations of the NI, effects of central microinfusions of relaxin‐3 or related agonist or antagonist ligands on physiology and behaviour, and the impact of relaxin‐3 gene deletion or knockdown. Although these individual studies reveal facets of the likely functional relevance of the NI and relaxin‐3 systems for human physiology and behaviour, the differences observed in responses between species (e.g. rat vs. mouse), the clearly identified heterogeneity of NI neurons and procedural differences between laboratories are some of the factors that have prevented a precise understanding of their function. This review aims to draw attention to the current preclinical evidence available that suggests the relevance of the NI/relaxin‐3 system to the pathology and/or symptoms of certain neuropsychiatric disorders and to provide cognizant directions for future research to effectively and efficiently uncover its therapeutic potential.
Linked Articles
This article is part of a themed section on Recent Progress in the Understanding of Relaxin Family Peptides and their Receptors. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.10/issuetoc
Abbreviations
- AD
Alzheimer's disease
- CRF
corticotrophin‐releasing factor
- EPM
elevated plus maze
- HC
hippocampal
- HP‐mPFC
hippocampo‐medial prefrontal cortical
- MRN
median raphe nucleus
- NI
nucleus incertus
- PVN
paraventricular nucleus of hypothalamus
- RPO
reticularis pontis oralis
- SHS
septohippocampal system
Tables of Links
| TARGETS | |
|---|---|
| GPCRs a | PAC1 receptor |
| 5‐HT1A receptor | RXFP3 receptor |
| CRF1 receptor | V1A receptor |
| CRF2 receptor | Y5 receptor |
| Dopamine D2 receptor | Nuclear hormone receptors b |
| Dopamine D3 receptor | Glucocorticoid receptor, NR3C1 |
| Neuropeptide S receptor |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,bAlexander et al., 2015b, 2015a).
Introduction
The nucleus incertus (NI)/relaxin‐3 system, highly conserved across species, has been studied for over a decade (Goto et al., 2001; Bathgate et al., 2002). The NI is heterogeneous, with anatomically distinct compacta and dissipata regions differing in electrophysiological properties (Nunez et al., 2006; Ma et al., 2013; Martinez‐Bellver et al., 2015) and expression of neuropeptides, neurotransmitters and receptors (Sutton et al., 2004; Tanaka et al., 2005; Miyamoto et al., 2008; Ryan et al., 2011; Kumar et al., 2015) including the neuropeptide, relaxin‐3, which participates in a variety of neurophysiological functions (see Ma et al., (in press)).
The NI/relaxin‐3 system in the rat robustly responds to stressors suggesting that it will be up‐regulated and potentially dysfunctional in disease states associated with excessive stress. Because neuropeptides are preferentially released under conditions of high neural tone which are more likely to be present in pathological states, neuropeptide receptor antagonists are likely to produce specific and effective actions to correct pathological disequilibrium. In addition, due to the neuromodulatory nature of neuropeptides, interfering with neuropeptide signalling is less likely to have overt, strong side effects than manipulations of classical neurotransmitter systems (Hokfelt et al., 2000). Similarly, we argue that studying a potentially dysregulated relaxin‐3 system in preclinical disease models could yield significant insights into its functional roles and simultaneously identify the therapeutic potential of inhibiting or enhancing relaxin‐3‐related signalling.
There is an exigent need to examine whether the acquired knowledge of this neuropeptide system (Ryan et al., 2011; Smith et al., 2011, 2014; Ma and Gundlach, 2015) can be translated from bench to bedside. However, the few human studies on the NI/relaxin‐3 system expose a disparity between the animal and human systems and reveal a clear need for more cognizant future studies in this field. Of course, caution will be required when extrapolating preclinical findings to design of human studies, but use of animal models of human diseases and disorders is still indispensable to drug discovery (Markou et al., 2009; Nestler and Hyman, 2010; Baldarelli, 2012). This review will briefly summarize findings linking the NI/relaxin‐3 system to neuropsychiatric conditions such as anxiety, depression, disorders related to appetite, stress and cognitive dysfunction (for more detailed reviews see Ryan et al., 2011; Smith et al., 2011; Ma and Gundlach, 2015) and will focus on providing a ‘road‐map’ for future research in preclinical disease models and fundamental clinical studies.
Stress
In humans, dysregulation of adaptive mechanisms with inappropriate and/or exaggerated responses to psychological stressors manifests as a disorder in itself and/or as a cluster of symptoms co‐morbid with other psychiatric conditions such as anxiety and depression (American Psychiatric Association, 2013; Gold, 2015). Preclinical studies that have successfully modelled mechanisms, such as plasticity and cognitive dysfunction related to stress, have furthered our understanding of the neurobiology of stress and related disorders and provided screening methods to identify new candidate drugs for treating these disorders (Kormos and Gaszner, 2013; Bock et al., 2015; Chattarji et al., 2015; Constantinof et al., 2015; Musazzi and Marrocco, 2016). Among the multitude of neuropeptides implicated in stress responses, the role of corticotropin‐releasing factor (CRF) is central (Aubry, 2013; Kormos and Gaszner, 2013). The conspicuous expression of the CRF1 receptor in the NI of the rat and its high co‐expression with relaxin‐3 (Potter et al., 1994; Chalmers et al., 1995; Bittencourt and Sawchenko, 2000; Tanaka et al., 2005; Ma et al., 2013), not only defined the topography of the NI but also inspired early studies to evaluate its role in stress responses (Figure 1) (Rivest et al., 1995; Tanaka et al., 2005).
Figure 1.
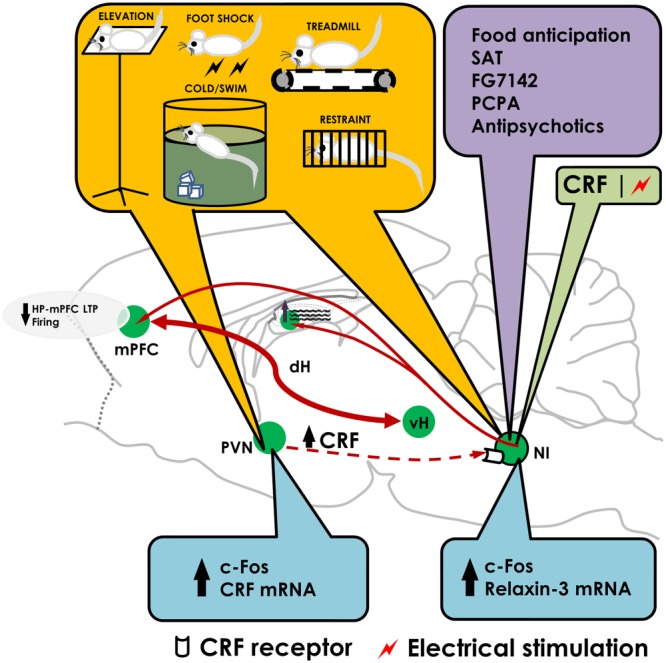
The nucleus incertus responds to stress and contributes to stress responses. The diagram summarises recent findings on the role of the nucleus incertus (NI) in stress. Stressors (yellow box) may directly or indirectly (activating the PVN and increasing CRF released) activate the NI as indicated by c‐Fos induction or enhanced expression of relaxin‐3 mRNA (blue box). Micro‐infusion of CRF or electrical stimulation of NI (green box) suppressed firing of mPFC neurons and LTP in the HP‐mPFC pathway. Stimulation of NI is also known to increase theta activity in the dorsal hippocampus. In addition, behavioural or pharmacological manipulations (purple box) such as food anticipation, exposure to spontaneous alternation tasks (SAT), pharmacological treatments (FG‐7142, PCPA and antipsychotics) have been shown to induce c‐Fos in the NI. dH: dorsal hippocampus; vH: ventral hippocampus; PCPA: para‐chlorophenylalanine.
Stress induced by exposure of rodents to different behavioural stressors, exogenous CRF in the brain (presumed to simulate a stressful situation) or treatment with anxiogenic agents such as the inverse benzodiazepine agonist, FG‐7142 (Lawther et al., 2015), resulted in up‐regulation of c‐Fos in the NI as well as other areas (Senba et al., 1993; Cullinan et al., 1995; Bittencourt and Sawchenko, 2000; Passerin et al., 2000; Dayas et al., 2001; Timofeeva et al., 2003; Tanaka et al., 2005; Cano et al., 2008; Rajkumar et al., 2016), and in some cases, increased relaxin‐3 expression (Banerjee et al., 2010; Lenglos et al., 2013; Lenglos et al., 2014), which was reversible by pretreatment with a CRF1 antagonist (Banerjee et al., 2010) (Figure 1). Human relaxin‐3 could have a direct stimulatory effect on CRF neurons as suggested by in vivo findings, in which i.c.v. infusion of human relaxin‐3‐augmented plasma corticosterone levels, increased plasma ACTH and prolactin and induced c‐Fos and CRF mRNA in the paraventricular nucleus of the hypothalamus (PVN) – a structure with a high density of the relaxin family peptide receptor RXFP3 (Figure 2) (Ma et al., 2007; Watanabe et al., 2011a; McGowan et al., 2014).
Figure 2.
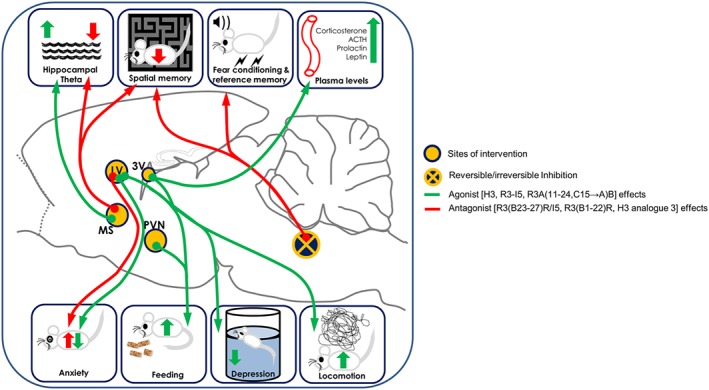
Pharmacological effects of the central administration of RXFP3 receptor ligands and inhibition of the NI. Agonist effects are shown in green and antagonist effects are shown in red. Infusion of relaxin‐3 or RXFP3 receptor agonists in the lateral ventricle (LV) causes increased feeding and locomotion, and decreased anxiety and depressive‐like behaviour. Infusion of relaxin‐3 into hypothalamic centres, especially the PVN, increased feeding behaviour. Infusion of relaxin‐3 into the third ventricle (3V) increased both feeding behaviour and plasma levels of corticosterone, ACTH, prolactin and leptin. Infusion of RXFP3 receptor antagonist in the medial septum (MS) impaired spatial memory and decreased hippocampal theta activity, whereas infusion of agonist in the MS increased hippocampal theta activity. Finally, reversible inhibition (by lidocaine) of the NI causes impairment in spatial and reference memory and irreversible inhibition (by CRF‐saporin or electrolytic lesions) of the NI causes derangements in fear conditioning.
We showed that CRF infusion into, or electrical stimulation of, the NI, to simulate a stressful situation or a condition with high neural activity tone, affected plasticity as measured by LTP in the hippocampo‐medial prefrontal cortical (HP‐mPFC) pathway (Farooq et al., 2013). Infusion of the CRF1 receptor antagonist antalarmin into the NI, prior to or after exposure of rats to elevation stress, reversed the elevation stress‐induced suppression of LTP in the HP‐mPFC pathway (Rajkumar et al., 2016). Together, these results demonstrate that the NI‐HP‐mPFC is a stress‐responsive circuit and the NI, especially via CRF1 receptor activation, contributes to stress‐induced impairment in neuronal plasticity that is likely to have implications in stress‐related cognitive dysfunction.
Behavioural activation
The NI innervates several areas that are involved in regulating levels of behavioural activity such as the reticularis pontis oralis (RPO), median raphe nucleus (MRN), interpeduncular nuclei and the lateral preoptic area (Goto et al., 2001). During stress and food anticipatory activity, NI activation is positively correlated with high behavioural activity leading to the hypothesis that the NI, together with the interpeduncular and median raphe nuclei, may function to control behavioural activation. Indeed, this circuit may be part of the behavioural inhibition system (McNaughton and Gray, 2000) centred on the septohippocampal system (SHS). This theory is based on the findings that all anxiolytics (typical and novel) increase the threshold of septal driving of hippocampal (HC) theta waves, reduce RPO‐induced theta waves and produce similar behavioural responses to lesions of the SHS. According to this theory, the hippocampus functions as a comparator, determining when expected outcomes do not meet actual outcomes and produces an output affecting attention, arousal and behavioural inhibition essentially giving rise to anxiety (McNaughton and Gray, 2000; McNaughton and Corr, 2004). Studies to date indicate that the main inputs to the hippocampus are the septal pacemaker neurons, pedunculopontine tegmental nucleus, amygdala, superior colliculus and substantia nigra, which provide input about signals of non‐reward, punishment, unconditioned or conditioned fear stimuli. As direct connections between the RPO and SHS are scarce (Vertes and Martin, 1988; Nunez et al., 1991), the RPO is thought to provide ascending control via the supramammillary nucleus during tasks such as the fixed interval schedule (McNaughton and Gray, 2000); and it is thought that the NI mediates the RPO influence on the SHS during tasks such as exploration, as inhibition of the NI abolished RPO stimulation‐induced HC theta waves (Nunez et al., 2006). The NI perhaps modulates anxiety by altering the input to the SHS and contributes to the stress‐responsive nature of this circuit (see Ma et al., (in press)).
Studies from our laboratories revealed that the NI/relaxin‐3 system may subserve behavioural activation. Infusion of a dopamine D2/D3 receptor agonist, quinpirole, into the NI consistently reduced locomotion in different behavioural paradigms (Kumar et al., 2015) demonstrating that activation of D2 receptors in the NI, that are highly co‐expressed with relaxin‐3 and CRF1 receptors, could modulate behavioural activity. Additionally, the role of D2 receptors may be modulated by stress as activation in a high, but not a low stress environment reduced feeding (Kumar et al., 2015). Furthermore, we observed a causal role for the NI in locomotion and behavioural activation in rats by employing high frequency electrical microstimulation of the NI via chronically implanted microelectrodes (Farooq et al., 2016). Microstimulation of the NI was sufficient to induce robust forward locomotion, rotational behaviour and behavioural activity. The latency of this effect indicated that the NI most likely induced locomotion via modulation of premotor areas rather than directly affecting motor cortices (Farooq et al., 2016). HC theta is strongly linked to locomotion in behaving animals and to processing of spatially‐related sensory input (Vanderwolf, 1969; Whishaw and Vanderwolf, 1973). Structures involved in modulating HC theta such as the RPO, supramammillary nucleus and raphe nuclei similarly, upon stimulation, evoke or inhibit locomotion accompanied by increased or decreased HC theta activity. It will therefore be important to concurrently measure HC theta activity and behavioural activation while stimulating the NI. A recent study using designer receptors exclusively activated by designer drugs (DREADDs) to selectively activate NI neurons caused cortical desynchronization, heightened arousal and increased locomotion as well as elevated vigilance behaviours in fear conditioning (Ma et al., 2016). These complementary results indicate that the NI/relaxin‐3 system is involved in stress‐related modulation of behavioural activity levels, a key feature underlying several functions dysregulated in various neuropsychiatric disorders and manifesting as ‘lethargy’ (Czeh et al., 2016).
Anxiety
Anxiety is a sustained state of vigilance marked by increased arousal and behavioural inhibition (Davis et al., 2010). Preclinical research on the mechanisms underlying anxiety places most emphasis on the role of the amygdala, bed nucleus of stria terminalis (BNST), ventral hippocampus (vHC) and prefrontal cortex (PFC) – all of which have moderate to strong connections with the NI (Goto et al., 2001; Olucha‐Bordonau et al., 2003; Santos et al., 2016).
Central infusions of RXFP3 receptor ligands suggest that relaxin‐3 signalling has an anxiolytic function physiologically (Figure 2) (Nakazawa et al., 2013; Ryan et al., 2013a) (see Ma et al., (in press)). Relaxin‐3 signalling has been shown to alter oxytocin levels in the hypothalamus (Ganella et al., 2013; Nakazawa et al., 2013). Oxytocin is a likely effector of relaxin‐3/RXFP3 receptor signalling based on the robust expression of these receptors in the hypothalamic nuclei synthesizing oxytocin, the PVN and supraoptic nucleus and the well‐researched role of oxytocin in modulating behavioural responses, such as anxiety, fear, social behaviour and stress responses (McCarthy et al., 1996; Carter, 2003; Slattery and Neumann, 2010; Viero et al., 2010) and neuropsychiatric diseases like autism and obsessive–compulsive disorder (Leckman et al., 1994; Hollander et al., 2003; Hollander et al., 2007).
Selective ablation of NI cells by CRF‐saporin administration (Lee et al., 2014) and electrolytic lesions caused deficits in fear conditioning, particularly in the extinction of conditioned fear, with no effect on fear acquisition or retrieval of extinction memory (Pereira et al., 2013). Extinction of learned fear features in post‐traumatic stress disorder, panic disorder and phobias and is impaired in rats bred for high anxiety (Anderson and Insel, 2006; Pull, 2007).
Studies of neuropeptides such as CRF, neuropeptide Y (NPY), arginine vasopressin (AVP), oxytocin and substance P highlight that standard behavioural tests are designed with neurotransmitters in mind and may not be the most suited to detect neuropeptide‐mediated changes in anxiety (Hokfelt et al., 2000; Rotzinger et al., 2010). In many cases, it is necessary to introduce stress as an external stimulus to observe neuropeptide‐induced behavioural effects (Heinrichs et al., 1994). This phenomenon has been demonstrated with regard to relaxin‐3/RXFP3 receptor signalling, whereby i.c.v. administration of RXFP3 agonist did not alter basal anxiety but attenuated FG7142‐induced elevated anxiety (Zhang et al., 2015); suggesting the relaxin‐3/RXFP3 receptor system may be activated in states of elevated anxiety. This is of therapeutic interest because, if the system is dysregulated in diseased states, modulating it may not affect baseline functioning, thus reducing side effects. Despite reports of apparent inconsistent phenotypes of relaxin‐3 knockout mice in tests of innate anxiety‐like behaviour (Smith et al., 2009; Watanabe et al., 2011a), studying how these knockout mice respond to treatments that are designed to induce stress‐induced anxiety may better reveal the importance of relaxin‐3 signalling.
Depression
Several neuropeptides have strong regulatory roles in the function of the hypothalamic–pituitary–adrenal (HPA) axis, which is dysfunctional in depressed patients (Rybakowski and Twardowska, 1999; Maletic et al., 2007; Pariante and Lightman, 2008). CRF, substance P, NPY, AVP and oxytocin all offer novel therapeutic possibilities for treatment of depression (Catena‐Dell'Osso et al., 2013), and several studies suggest relaxin‐3 may influence the HPA axis (McGowan et al., 2008; McGowan et al., 2014). Further, reduced neurogenesis in the dentate gyrus has also been implicated in depression, as patients have smaller hippocampus volumes, and neurogenesis is required for effective antidepressant action in preclinical models (Santarelli et al., 2003; Small et al., 2011). Additionally, maturation of new neurons may explain the time lag in antidepressant action (Malberg et al., 2000). Interestingly, relaxin‐3 fibres are present in the dentate gyrus, and our unpublished data indicate that neurogenesis and neuronal maturation is perturbed in an age‐, sex‐ and septotemporal axis‐dependent manner in relaxin‐3 knockout mice (Dawe and Gundlach laboratories), indicating a possible involvement of relaxin‐3 in regulation of adult neurogenesis in hippocampus and depression. In the forced swim test, central infusion of a RXFP3 receptor agonist reduced immobility (Ryan et al., 2013a), while one strain of relaxin‐3 knockout mice displayed increased immobility (Smith et al., 2009), indicating that relaxin‐3 has an antidepressant effect. However, in another relaxin‐3 knockout strain, forced swim test behaviour was unaltered (Watanabe et al., 2011b). These results highlight the pressing need for detailed behavioural phenotyping of knockout strains in a series of antidepressant assays including the tail suspension, social interaction and sucrose preference tests, as well as sleep EEG studies.
PET studies conducted in patients of major depressive disorder consistently reveal widespread reductions in binding to 5‐HT1A receptors in the raphe, limbic and cortical regions (Savitz and Drevets, 2013; Kohler et al., 2016). In this regard, depletion of 5‐HT in rats, by p‐chlorophenylalanine (fenclonine) administration, nearly doubled relaxin‐3 mRNA in the NI and these neurons express 5‐HT1A receptor‐like immunoreactivity (Miyamoto et al., 2008). Thus, dysregulation of 5‐HT in depression may be accompanied by altered relaxin‐3 tone, which could contribute to changes in sleep, appetite, mood or stress responses. Infusion of an RXFP3 receptor antagonist, both i.c.v. and intra‐BNST, prevented stress‐induced alcohol relapse in alcohol‐preferring rats (Ryan et al., 2013a) (see Ma et al., (in press)), linking relaxin‐3 signalling to stress and reward, perhaps similar to that in depression where uncontrollable stress usually acts as a trigger and anhedonia is a symptom. Depression is also characterized by disturbances in appetite, as discussed below.
Appetite regulation
Appetite, which is regulated by central and peripheral mechanisms (Gao and Horvath, 2007; Lenard and Berthoud, 2008; Keen‐Rhinehart et al., 2013), is dysregulated in various disorders such as depression, schizophrenia and anxiety, which are associated with derangements in levels of neurotransmitters (dopamine and 5‐HT) and neuropeptides, including insulin, ghrelin, agouti‐related protein, β‐endorphin (proopiomelanocortin), orexins and NPY.
There is good evidence that relaxin‐3 is an orexigenic peptide in the rat. Acute and chronic central administration of relaxin‐3 or RXFP3 receptor agonists consistently induced hyperphagia, accompanied by weight gain and metabolic changes (Liu et al., 2005; McGowan et al., 2005; Hida et al., 2006; Kuei et al., 2007; Liu et al., 2009; Sutton et al., 2009; Shabanpoor et al., 2012; Ganella et al., 2013; Hossain et al., 2013). This is likely to be due to actions in hypothalamic areas such as the paraventricular, supraoptic and arcuate nuclei and the anterior preoptic area, as direct peptide injections into these areas elicits a robust orexigenic effect (McGowan et al., 2006; McGowan et al., 2007).
Weight gain is a common side effect of antipsychotic drug treatments, and acute treatment of rats with antipsychotics produced a distinct pattern of c‐Fos expression in appetite‐related neuronal structures namely, arcuate nucleus, PVN and paraventricular thalamic nucleus, and in the NI, suggesting it may play a role in acute antipsychotic‐induced hyperphagia (Rajkumar et al., 2013). An orexigenic action of relaxin‐3 in the hypothalamus thus presents a paradigm in which to explore effects of selective RXPF3 antagonists for treatment of appetite disorders, including management of antipsychotic drug‐induced weight gain and hyperphagia associated with depression.
Cognition
HC theta activity is strongly associated with memory consolidation, arousal, behavioural inhibition, anxiety, sleep states, exploration and movement (Bland, 1986; Bland and Bland, 1986; Vinogradova, 1995; McNaughton and Gray, 2000; Vertes et al., 2004; Vertes, 2005; Buzsaki and Moser, 2013; McNaughton et al., 2013). Anatomical connections suggest that the NI can influence rat HC theta rhythm via several pathways, including bidirectional connections to (i) medial septum/vertical limb of the diagonal band [later identified to be both GABAergic (inhibitory) and glutamatergic (excitatory) in nature] (Ma et al., 2007; Cervera‐Ferri et al., 2012) and hippocampus; (ii) RPO, a brainstem ‘generator’ of HC theta waves; (iii) habenula and interpeduncular nucleus, known to control HC theta rhythm (Valjakka et al., 1998); (iv) MRN, involved in desynchronizing theta rhythm (Kinney et al., 1995; Vertes and Kocsis, 1997; Viana Di Prisco et al., 2002); and (v) posterior hypothalamus (Bland et al., 1994; Oddie et al., 1994).
The NI/relaxin‐3 system has been shown to induce (Nunez et al., 2006), display coherence with (Cervera‐Ferri et al., 2011) and fire in a phase‐locked manner with (Ma et al., 2013) HC theta oscillations and modulate relevant behavioural functions such as spatial memory (Ma et al., 2009; Albert‐Gasco et al., 2016) (see Ma et al., (in press)). Interestingly, tetanic stimulation of the NI in freely‐moving rats evoked robust forward locomotion at latencies consistent with its modulation of premotor areas such as the SHS and, probably, HC theta activity (Farooq et al., 2016).
We have explored the contribution of NI to the plasticity in the HP‐mPFC pathway. Firstly, it was demonstrated that CRF1/2 receptor‐like immunoreactivity was present in neurons of the NI that project to the mPFC (Hoover and Vertes, 2007; Farooq et al., 2013). Secondly, electrical stimulation of the NI suppressed spontaneous firing of mPFC neurons, as reflected by a decreased firing rate, and infusion of CRF to the NI suppressed the firing rate and burst firing observed (Farooq et al., 2013). Thirdly, high frequency stimulation of NI or exogenous CRF administration into the NI attenuated LTP in the HP‐mPFC pathway (Farooq et al., 2013). Furthermore, inactivation of NI with lidocaine, prior to high frequency stimulation of the perforant path, affected LTP induction in the dentate gyrus (Nategh et al., 2016). These reports, along with the finding that direct infusion of antalarmin into the NI reverses elevation stress‐induced suppression of LTP in the HP‐mPFC pathway (Rajkumar et al., 2016), reveal that CRF1 receptor‐expressing neurons in the NI contribute to synaptic plasticity in the HP‐mPFC pathway and via connections to the mPFC may affect cognitive processes such as spatial, working and fear memory, under stress‐related conditions (Granon and Poucet, 1995; Seamans et al., 1995; Delatour and Gisquet‐Verrier, 1996; Laroche et al., 2000; Burgos‐Robles et al., 2009).
Future directions
Better targeting of RXFP3 receptors and novel peptide and drug development
The lack of a broad range of molecular tools to target RXFP3 receptors, including non‐peptide agonists and antagonists that penetrate the blood–brain barrier seriously impedes behavioural neuroscience research on the relaxin‐3/RXFP3 receptor system. As discussed, the NI/relaxin‐3 system is a prospective target for pharmaceutical intervention in a range of neuropsychiatric disorders, and thus, suitable ligands active at RXFP3 receptors are crucial. Currently available RXFP3 receptor agonists are human relaxin‐3, R3/I5 (Haugaard‐Jonsson et al., 2008) and R3A(11–24,C15 → A)B (human relaxin‐3 analogue 2); RXFP3‐A2 (Shabanpoor et al., 2012), while the available antagonists are R3(BΔ23–27)R/I5 (Kuei et al., 2007), R3(B1–22)R (Haugaard‐Kedstrom et al., 2011) and human relaxin‐3 analogue 3; RXFP3‐R3 (Shabanpoor et al., 2012) (Table 1) (see Patil et al., (in press)). Stapling of peptides, whereby peptides are chemically stabilized by crosslinking with small molecules, has been proposed as a highly effective method of improving the “druggability” of peptides (Verdine and Walensky, 2007; Jubb et al., 2012; Higueruelo et al., 2013; Walensky and Bird, 2014). Recent advances in stapling relaxin‐3 B chain analogues (Hojo et al., 2016; Jayakody et al., 2016) suggest that stapling may hold promise for development of more ‘druggable’ peptide ligands for the RXFP3 receptor (see Patil et al., (in press)).
Table 1.
Effects of intra‐cerebral microinfusion of RXFP3 receptor ligands
| Ligand | Infusion site | Infusion type | Species | Effects observed | References |
|---|---|---|---|---|---|
| Agonists | |||||
| Human relaxin‐3 | i.c.v. (lateral ventricle) | Acute | Rat | Sex‐specific increase in feeding with higher food intake induction in females. | (Calvez et al., 2015) |
| Human relaxin‐3 | i.c.v. (lateral ventricle) | Acute | Rat | Increased food intake in female but not male rats. Increased plasma corticosterone in male but not female rats. Increased CRF and c‐fos mRNA in the parvocellular PVN of male but not female rats. Increased CRF mRNA in the BNST of female but not male rats. | (Lenglos et al., 2015) |
| Human relaxin‐3 | i.c.v. (lateral ventricle) | Acute | Rat | Decreased anxiety behaviour in elevated plus maze and shock probe‐burying test. Increased locomotion in novel environment indicating reduced stress response. | (Nakazawa et al., 2013) |
| Human relaxin‐3 | i.c.v. (lateral ventricle) | Acute | Rat | Increased plasma ACTH. Increased c‐fos and CRF mRNA in PVN. | (Watanabe et al., 2011a) |
| Human relaxin‐3 | i.c.v. (lateral ventricle) | Acute | Rat | Increased food intake 1 h after infusion. | (McGowan et al., 2007) |
| Human relaxin‐3 | i.c.v. (lateral ventricle) | Chronic (osmotic minipump for 14 days) | Rat | Increased food consumption and weight gain. Plasma concentrations of leptin and insulin increased. | (Hida et al., 2006) |
| Human relaxin‐3 | i.c.v. (third ventricle) | Acute | Rat | Increased plasma corticosterone. | (McGowan et al., 2014) |
| Human relaxin‐3 | i.c.v. (third ventricle) | Acute | Rat | Increased food intake 1 h after infusion in satiated rats in early light/dark phase. | (McGowan et al., 2005) |
| Human relaxin‐3 | PVN | Acute | Rat | Increased plasma ACTH, corticosterone and prolactin. | (McGowan et al., 2014) |
| Human relaxin‐3 | PVN | Subchronic (twice daily for 7 days) | Rat | Increased cumulative food intake. Plasma leptin increased. Plasma thyroid stimulating hormone was decreased. | (McGowan et al., 2007) |
| Human relaxin‐3 | PVN | Acute | Rat | Increased food intake 1 h after infusion. Plasma thyroid stimulating hormone was decreased. | (McGowan et al., 2006) |
| Human relaxin‐3 | PVN | Acute | Rat | Increased food intake 1 h after infusion in satiated rats in early light/dark phase. | (McGowan et al., 2006) |
| Human relaxin‐3 analogue 2 | i.c.v. (lateral ventricle) | Acute | Rat | Increased food intake 1 h after infusion in satiated rats in early light phase. | (Shabanpoor et al., 2012) |
| R3/I5 | i.c.v. (lateral ventricle) | Acute | Rat | Dose‐dependent increase in locomotion. | (Sutton et al., 2009) |
| R3/I5 | i.c.v. (lateral ventricle) | Chronic (osmotic minipump for 14 days) | Rat | Increased food intake and body weight. Increased epididymal fat, plasma insulin, leptin, adiponectin, plasma testosterone and angiotensinogen. Decreased growth hormone. | (Sutton et al., 2009) |
| R3/I5 | PVN | Chronic (rAAV expression of R3/I5 for 8 weeks) | Rat | Increase in daily food intake and body weight gain. | (Ganella et al., 2013) |
| R3/I5 | Medial septum | Acute | Rat | Increase HC theta power. | (Ma et al., 2009) |
| R3A(11–24,C15 → A)B | i.c.v. (lateral ventricle) | Acute | Mouse | Reduced elevated anxiety induced by FG7142 in light dark box and social interaction test. | (Zhang et al., 2015) |
| R3A(11–24,C15 → A)B | i.c.v. (lateral ventricle) | Acute | Rat | Decreased anxiety behaviour in EPM and light/dark box. Decreased depressive‐like behaviour in forced swim test in pretested rats but not experimentally naïve rats. | (Ryan et al., 2013a) |
| Antagonists | |||||
| R3(B1–22)R | i.c.v. (lateral ventricle) | Acute | Mouse | Increased anxiety behaviour in EPM. | (Zhang et al., 2015) |
| Human relaxin‐3 analogue 3 | i.c.v.(lateral ventricle) | Acute | Rat | Blocked increase in food intake by H3 analogue 2. | (Shabanpoor et al., 2012) |
| R3(B23–27)R/I5 | i.c.v. (lateral ventricle) | Chronic (osmotic minipump for 14 days) | Rat | Plasma growth hormone decreased. | (Sutton et al., 2009) |
| R3(B23–27)R/I5 | Medial septum | Acute | Rat | Decreased HC theta power. Impairs spatial working memory performance in spontaneous alternation task. | (Ma et al., 2009) |
| R3(B23–27)R/I5 | i.c.v. (lateral ventricle) | Acute | Rat | Blocks increase in food intake induced by agonist R3/I5. | (Kuei et al., 2007) |
Preclinical translational studies
The key avenues of preclinical research in this area are to identify (1) regulation of other neurotransmitter systems by relaxin‐3; (2) interplay of second messenger systems due to interactive effects of relaxin‐3, GABA and CRF in the NI neurons; (3) real‐time patterns of NI relaxin‐3 neuron firing during home cage activity and on exposure to stressors; (4) effects of selective optogenetic stimulation/suppression of NI relaxin‐3 neurons in behaving rodents; (5) effects of stimulation/suppression of NI neurons (including specific populations) on cortical EEG and theta wave rhythms; (6) use of DREADDs (Roth, 2016) to target relaxin‐3 and/or CRF1 receptor positive neurons to understand the effects of acute and chronic activation/suppression of the NI neurons (Ma et al., 2016); and (7) measuring expression levels of target proteins or high resolution MRI of the NI during basal conditions and in response to pharmacological intervention especially, with drugs and ligands implicated in neuropsychiatric conditions.
Importantly, research so far has been largely limited to the investigation of NI neurons and relaxin‐3/RXFP3 receptor signalling in normal rodents. Thus, further research on the outcomes of disease pathologies in relevant preclinical disease models on NI neurons and the relaxin‐3 system will be crucial to understand its full physiological role more clearly and its therapeutic potential as mapped out below.
Models of anxiety and depression
High anxiety (HAB) rats, selectively bred based on elevated plus maze (EPM) performance, display hyper‐emotionality, hyper‐reactivity of the HPA axis, impaired fear extinction, passive coping strategies and stronger responses to anxiolytics and antidepressants (Liebsch et al., 1998; Henniger et al., 2000; Ohl et al., 2001; Wigger et al., 2001; Landgraf and Wigger, 2002; Keck et al., 2003), suggesting they effectively model co‐morbid depression and anxiety, commonly observed in the clinic (Landgraf et al., 1999; Keck et al., 2001). AVP expression is elevated in the PVN of HAB rats compared with their counterpart low anxiety rats and is functionally relevant as intra‐PVN infusion of a V1A receptor antagonist produced an anxiolytic effect in HAB rats (Wigger et al., 2004). CRF expression in the BNST is reduced, and CRF2 receptor binding in hypothalamic nuclei and amygdala is increased in HAB rats (Wigger et al., 2004). Though not entirely consistent, previous results indicate that the NI/relaxin‐3 system has an anxiolytic effect, and it is possible that there is reduced relaxin‐3 expression and concentrations in the NI and/or key target regions like the amygdala and BNST in HAB rats.
Similarly, the Flinders sensitive line (FSL) of rats were developed through selective breeding to exhibit cholinergic supersensitivity, a feature seen in depressed patients (Overstreet, 1993). FSL rats display high levels of immobility in the forced swim test, low social interaction, altered appetite and weight, impaired response to reward, lethargy and particularly strong stress responses, such as increased anhedonia in response to a chronic mild stress and behavioural inhibition after a foot shock (Overstreet et al., 1995). Reduced NPY expression in the hippocampus of FSL rats indicates its involvement in the pathophysiology of depression (Jimenez Vasquez et al., 2000). As relaxin‐3 neurons are stress‐responsive, FSL rats may display an altered profile of relaxin‐3 signalling and provide insights into a possible therapeutic opportunity in treating depression symptoms. Furthermore, because relaxin‐3 signalling has been strongly implicated in stress‐induced alcohol relapse (Ryan et al., 2013b) (see Ma et al., (in press)), the fawn‐hooded rat, which is a model for co‐morbid depression and alcoholism displaying high immobility on the forced swim test and high voluntary ethanol intake, is also worth considering for relaxin‐3 system studies (Overstreet et al., 2007; Rezvani et al., 2002).
In addition to these life‐long models created by selective breeding, more convenient models with greater construct validity may be those induced by environmental behavioural stressors. The chronic mild stress (CMS) model of depression, which is preferred for rats but has also been used in mice, is extensively validated and produces enduring changes in behaviour, neurotransmitter and hormonal concentrations and the immune system (Overstreet, 2012; Abelaira et al., 2013; Czeh et al., 2016). Notably, a similar 8‐week chronic stress regime imposed on relaxin‐3 knockout mice resulted in a sustained loss of body weight, but no marked changes in the forced swim test (Smith et al., 2009). Further studies in this area are warranted, including further tests of depressive phenotypes such as those revealed by the sucrose/saccharin preference test, because such changes are a primary characteristic of mice undergoing CMS (Katz, 1982; Willner, 2005).
Furthermore, a model of post‐traumatic stress disorder (PTSD) symptoms developed by exposing rats to ‘chronic plus acute prolonged stress’ treatment (Green et al., 2011) was reported to increase basal anxiety and fear responding while impairing extinction of learned fear and altering stress coping styles from active to passive, reducing HPA reactivity to acute stressors and reducing glucocorticoid receptor expression in the mPFC (Roth et al., 2012). Therefore, alterations in expression of relaxin‐3 in the NI or RXFP3 receptors in regions such as the amygdala, PFC, BNST and vHC could be examined in this model. If changes were observed, experiments could be designed to examine the ability of RXFP3 receptor agonists and antagonists to improve or worsen behavioural phenotypes and identify their main sites of action.
Need for clinical studies
While the vast majority of the experimental studies of the NI/relaxin‐3 system have been preclinical, there have been some efforts to examine links between relaxin‐3 signalling and metabolic and psychiatric disorders in patients, and the substantial body of evidence supporting important roles for this neuropeptide demands targeted translational studies. It is perhaps prudent to base any systematic clinical investigations of the relaxin‐3/RXFP3 system on successful studies linking other neuropeptide and neuropeptide receptor systems to neuropsychiatric disorders. These have largely been through molecular genetic studies combined with assays of peptide content in CSF, blood serum and/or post mortem brain samples from relevant patient groups. Recently, the serum levels of circulating pituitary adenylate cyclase activating polypeptide neuropeptide were positively correlated with PTSD symptoms only in women, not men (Ressler et al., 2011). A single nucleotide polymorphism in the PAC1 receptor gene could predict PTSD in women but not men and was associated with elevated reactivity of the amygdala and hippocampus to threatening stimuli as well as lower functional connectivity between the two structures studied via functional MRI (Stevens et al., 2014). Similarly, a single nucleotide polymorphism of the neuropeptide S receptor resulting in the mutated T‐allele was found to be associated with panic disorder, increased sensitivity to anxiety, greater stress response, a hyperactive HPA axis and increased activity of the basolateral amygdala when exposed to stress (Kumsta et al., 2013). NPY expression has been found to be reduced in the caudate nucleus and frontal cortex of suicide victims and in the CSF of depressed patients (Widerlov et al., 1988; Widdowson et al., 1992) and blood samples from PTSD patients (Sah et al., 2009). Currently, intranasal infusion of NPY is being developed and tested preclinically as a treatment for PTSD, to enable rapid delivery to the brain and avoid the side effects of peripheral administration (Serova et al., 2013; Sabban et al., 2016).
In the first studies of this type related to the relaxin‐3/RXFP3 receptor system, polymorphisms in the relaxin‐3 and RXFP3, RXFP4 receptor genes were reported to be associated with hypercholesterolemia, obesity and diabetes in patients being treated with antipsychotic drugs, corroborating a likely role of the relaxin‐3 system in metabolism and regulation of appetite and body weight (Munro et al., 2012). In separate studies, serum relaxin‐3 levels were reported to be elevated in female patients with metabolic syndrome (Ghattas et al., 2013) but unaffected in patients with diabetes (Zhang et al., 2013), although these studies did not fully demonstrate that their assays specifically detected the relaxin‐3 peptide. However, if these studies are reflective of the existence of detectable levels of relaxin‐3 in the bloodstream, it might be prudent to examine the profile of relaxin‐3 levels in patients with eating disorders and/or appetite irregularities associated with neuropsychiatric diseases such as schizophrenia and depression (Nestler et al., 2002; Newcomer, 2007; Lungu et al., 2013).
Interestingly, in a gene association study of familial schizophrenia, a mutation in chromosome 5p locus was identified, and the RXFP3 receptor gene is located on this chromosome (Bespalova et al., 2005). Based on preclinical data, the relaxin‐3 system could be linked to the cognitive impairment as well as the negative symptoms of schizophrenia. While this is only an anecdotal observation at present, with the rapid increase in genetic information available from different patient groups, due to improvements in and reduced costs of gene sequencing, more detailed information about the profile of RXFP3 receptors in neuropsychiatric disorders should become available.
In a recent study of the neocortex of patients with Alzheimer's disease (AD) and their age‐matched controls, we have found that alterations in the levels of RXFP1 and RXFP3 receptors were more closely associated with depressive symptoms than with cognitive decline or Aβ42 levels (Lee et al., 2016). While RXFP3 receptor‐like immunoreactivity in the parietal cortex was up‐regulated in depressed AD patients and unchanged in non‐depressed AD patients, RXFP1 receptor‐like immunoreactivity in the parietal cortex was unchanged in depressed AD patients and down‐regulated in non‐depressed AD patients (Lee et al., 2016). Neuropsychiatric conditions such as depression, anxiety and psychosis in AD patients (also known as ‘behavioural and psychological symptoms of dementia’) are thought to arise from degeneration of monoaminergic neurons and accompanying perturbations in neurotransmission (Francis et al., 2010; Ramirez et al., 2014). Given the proposed role for relaxin‐3 in regulating monoaminergic transmission (Miyamoto et al., 2008), the relaxin‐3/RXFP3 receptor system may represent a novel target for treating neuropsychiatric symptoms. Previous reports suggest that dysregulation of the HPA axis in AD could underlie neuropsychiatric behaviours (Notarianni, 2013; Lucassen et al., 2014). Exogenous relaxin‐3 altered activity of the HPA axis and it is possible that changes in endogenous relaxin‐3/RXFP3 receptor signalling contribute to altered HPA axis functioning and adaptive plasticity in AD (Lee et al., 2016), although further studies are required to understand this relationship and confirm its existence in human brain.
Strong evidence demonstrates an association between the disruption of synchronous brain neural oscillations, which can be studied non‐invasively, and neuropsychiatric diseases (Buzsaki and Watson, 2012; Uhlhaas and Singer, 2012). Theta and alpha‐band activities evoked during sensorimotor gating are significantly reduced in schizophrenia patients, as well as their first‐degree relatives (Hong et al., 2008). A similar association exists between alterations of rhythmic activity and depression, bipolar disorder and autism spectrum disorders (Buzsaki and Watson, 2012). In this regard, the lack of direct connections of the RPO and other brainstem modulating structures to the SHS indicates that the NI relaxin‐3 signalling may provide an important relay centre for brainstem‐initiated signals driving HC theta activity and may play a causal role in the disruption of theta activity associated with neuropsychiatric diseases.
Brain imaging is a technique that has provided detailed macroscale information about the distribution and magnitude of neurotransmitter systems. For instance, using PET and single‐photon emission computed tomography tracers, dopamine transporter density has been found to be significantly reduced in the basal ganglia of depressed patients (Nutt, 2006). Although not tested in humans yet, PET tracers have been developed and tested successfully preclinically for Y5 receptors, which is linked to the pathophysiology of depression, anxiety and obesity (Hostetler et al., 2011; Kumar et al., 2016). Similarly, a labelled ligand specific for RXFP3 receptors would enable brain imaging of the densities of these receptors in patients.
Thus, it may be informative to conduct studies of relaxin‐3 levels in the CSF (blood) and genetic variants of relaxin‐3/RXFP3 receptors in both control subjects and patients with neuropsychiatric disorders to build a data set that might allow further rationally designed studies of causative associations. Comprehensive studies of relaxin‐3 and RXFP3 receptor concentrations in post mortem brain samples from key brain regions important in emotional regulation such as amygdala, hippocampus and BNST from these populations may also provide insights, particularly if the identity of the RXFP3 receptor‐expressing neurons in the different regions can be discovered.
In conclusion, it is hoped that the substantial preclinical evidence for the broad neurophysiological actions of relaxin‐3/RXFP3 receptor signalling and the growing momentum of research in the area will encourage more basic and clinical researchers to consider this conserved neuromodulatory system in their investigations.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The research completed by the authors' laboratories reviewed here was enabled by the Biomedical Research Council of Singapore (BMRC 10/1/21/19/645), the National Medical Research Council of Singapore (NMRC/1287/2011), the NMRC NUHS Centre Grant – Neuroscience Phenotyping Core (NMRC/CG/013/2013) and the National Health and Medical Research Council of Australia (Grants 1005988, 1067522 and 1106330). The authors wish to thank Dr Francis Tan Chee Kuan, Dr Corinne Lee Liying and Mr Farooq Usman for insightful scientific discussion and Mr Ho Woon Fei for excellent technical and administrative assistance.
Kumar, J. R. , Rajkumar, R. , Jayakody, T. , Marwari, S. , Hong, J. M. , Ma, S. , Gundlach, A. L. , Lai, M. K. P. , and Dawe, G. S. (2017) Relaxin’ the brain: a case for targeting the nucleus incertus network and relaxin‐3/RXFP3 system in neuropsychiatric disorders. British Journal of Pharmacology, 174: 1061–1076. doi: 10.1111/bph.13564.
References
- Abelaira HM, Reus GZ, Quevedo J (2013). Animal models as tools to study the pathophysiology of depression. Rev Bras Psiquiatr 35 (Suppl 2): S112–S120. [DOI] [PubMed] [Google Scholar]
- Albert‐Gasco H, Garcia‐Aviles A, Moustafa S, Sanchez‐Sarasua S, Gundlach AL, Olucha‐Bordonau FE et al. (2016). Central relaxin‐3 receptor (RXFP3) activation increases ERK phosphorylation in septal cholinergic neurons and impairs spatial working memory. Brain Struct Funct. doi: 10.1007/s00429-00016-01227-00428. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 17: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Press: Arlington, VA. [Google Scholar]
- Anderson KC, Insel TR (2006). The promise of extinction research for the prevention and treatment of anxiety disorders. Biol Psychiatry 60: 319–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry JM (2013). CRF system and mood disorders. J Chem Neuroanat 54: 20–24. [DOI] [PubMed] [Google Scholar]
- Baldarelli RM (2012). Drug discovery: In defence of the animal model. Nature 485: 309. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Shen PJ, Ma S, Bathgate RA, Gundlach AL (2010). Swim stress excitation of nucleus incertus and rapid induction of relaxin‐3 expression via CRF1 activation. Neuropharmacology 58: 145–155. [DOI] [PubMed] [Google Scholar]
- Bathgate RA, Samuel CS, Burazin TC, Layfield S, Claasz AA, Reytomas IG et al. (2002). Human relaxin gene 3 (H3) and the equivalent mouse relaxin (M3) gene. Novel members of the relaxin peptide family. J Biol Chem 277: 1148–1157. [DOI] [PubMed] [Google Scholar]
- Bespalova IN, Angelo GW, Durner M, Smith CJ, Siever LJ, Buxbaum JD et al. (2005). Fine mapping of the 5p13 locus linked to schizophrenia and schizotypal personality disorder in a Puerto Rican family. Psychiatr Genet 15: 205–210. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Sawchenko PE (2000). Do centrally administered neuropeptides access cognate receptors?: an analysis in the central corticotropin‐releasing factor system. J Neurosci 20: 1142–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland BH (1986). The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol 26: 1–54. [DOI] [PubMed] [Google Scholar]
- Bland BH, Oddie SD, Colom LV, Vertes RP (1994). Extrinsic modulation of medial septal cell discharges by the ascending brainstem hippocampal synchronizing pathway. Hippocampus 4: 649–660. [DOI] [PubMed] [Google Scholar]
- Bland SK, Bland BH (1986). Medial septal modulation of hippocampal theta cell discharges. Brain Res 375: 102–116. [DOI] [PubMed] [Google Scholar]
- Bock J, Wainstock T, Braun K, Segal M (2015). Stress In Utero: Prenatal Programming of Brain Plasticity and Cognition. Biol Psychiatry 78: 315–326. [DOI] [PubMed] [Google Scholar]
- Burgos‐Robles A, Vidal‐Gonzalez I, Quirk GJ (2009). Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci 29: 8474–8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Moser EI (2013). Memory, navigation and theta rhythm in the hippocampal‐entorhinal system. Nat Neurosci 16: 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Watson BO (2012). Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin Neurosci 14: 345–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvez J, Lenglos C, de Avila C, Guevremont G, Timofeeva E (2015). Differential effects of central administration of relaxin-3 on food intake and hypothalamic neuropeptides in male and female rats. Genes Brain Behav 14: 550–563. [DOI] [PubMed] [Google Scholar]
- Cano G, Mochizuki T, Saper CB (2008). Neural circuitry of stress‐induced insomnia in rats. J Neurosci 28: 10167–10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS (2003). Developmental consequences of oxytocin. Physiol Behav 79: 383–397. [DOI] [PubMed] [Google Scholar]
- Catena‐Dell'Osso M, Fagiolini A, Marazziti D, Baroni S, Bellantuono C (2013). Non‐monoaminergic targets for the development of antidepressants: focus on neuropeptides. Mini Rev Med Chem 13: 2–10. [PubMed] [Google Scholar]
- Cervera‐Ferri A, Guerrero‐Martinez J, Bataller‐Mompean M, Taberner‐Cortes A, Martinez‐Ricos J, Ruiz‐Torner A et al. (2011). Theta synchronization between the hippocampus and the nucleus incertus in urethane‐anesthetized rats. Exp Brain Res 211: 177–192. [DOI] [PubMed] [Google Scholar]
- Cervera‐Ferri A, Rahmani Y, Martinez‐Bellver S, Teruel‐Marti V, Martinez‐Ricos J (2012). Glutamatergic projection from the nucleus incertus to the septohippocampal system. Neurosci Lett 517: 71–76. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB (1995). Localization of novel corticotropin‐releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci 15: 6340–6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattarji S, Tomar A, Suvrathan A, Ghosh S, Rahman MM (2015). Neighborhood matters: divergent patterns of stress‐induced plasticity across the brain. Nat Neurosci 18: 1364–1375. [DOI] [PubMed] [Google Scholar]
- Constantinof A, Moisiadis VG, Matthews SG (2015). Programming of stress pathways: a transgenerational perspective. J Steroid Biochem Mol Biol 160: 175–180. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ (1995). Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64: 477–505. [DOI] [PubMed] [Google Scholar]
- Czeh B, Fuchs E, Wiborg O, Simon M (2016). Animal models of major depression and their clinical implications. Prog Neuropsychopharmacol Biol Psychiatry 64: 293–310. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C (2010). Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35: 105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA (2001). Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci 14: 1143–1152. [DOI] [PubMed] [Google Scholar]
- Delatour B, Gisquet‐Verrier P (1996). Prelimbic cortex specific lesions disrupt delayed‐variable response tasks in the rat. Behav Neurosci 110: 1282–1298. [DOI] [PubMed] [Google Scholar]
- Farooq U, Kumar JR, Rajkumar R, Dawe GS (2016). Electrical microstimulation of the nucleus incertus induces forward locomotion and rotation in rats. Physiol Behav 160: 50–58. [DOI] [PubMed] [Google Scholar]
- Farooq U, Rajkumar R, Sukumaran S, Wu Y, Tan WH, Dawe GS (2013). Corticotropin‐releasing factor infusion into nucleus incertus suppresses medial prefrontal cortical activity and hippocampo‐medial prefrontal cortical long‐term potentiation. Eur J Neurosci 38: 2516–2525. [DOI] [PubMed] [Google Scholar]
- Francis PT, Ramirez MJ, Lai MK (2010). Neurochemical basis for symptomatic treatment of Alzheimer's disease. Neuropharmacology 59: 221–229. [DOI] [PubMed] [Google Scholar]
- Ganella DE, Callander GE, Ma S, Bye CR, Gundlach AL, Bathgate RA (2013). Modulation of feeding by chronic rAAV expression of a relaxin‐3 peptide agonist in rat hypothalamus. Gene Ther 20: 703–716. [DOI] [PubMed] [Google Scholar]
- Gao Q, Horvath TL (2007). Neurobiology of feeding and energy expenditure. Annu Rev Neurosci 30: 367–398. [DOI] [PubMed] [Google Scholar]
- Ghattas MH, Mehanna ET, Mesbah NM, Abo‐Elmatty DM (2013). Relaxin‐3 is associated with metabolic syndrome and its component traits in women. Clin Biochem 46: 45–48. [DOI] [PubMed] [Google Scholar]
- Gold PW (2015). The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry 20: 32–47. [DOI] [PubMed] [Google Scholar]
- Goto M, Swanson LW, Canteras NS (2001). Connections of the nucleus incertus. J Comp Neurol 438: 86–122. [DOI] [PubMed] [Google Scholar]
- Granon S, Poucet B (1995). Medial prefrontal lesions in the rat and spatial navigation: evidence for impaired planning. Behav Neurosci 109: 474–484. [DOI] [PubMed] [Google Scholar]
- Green MK, Rani CS, Joshi A, Soto‐Pina AE, Martinez PA, Frazer A et al. (2011). Prenatal stress induces long term stress vulnerability, compromising stress response systems in the brain and impairing extinction of conditioned fear after adult stress. Neuroscience 192: 438–451. [DOI] [PubMed] [Google Scholar]
- Haugaard‐Jonsson LM, Hossain MA, Daly NL, Bathgate RA, Wade JD, Craik DJ et al. (2008). Structure of the R3/I5 chimeric relaxin peptide, a selective GPCR135 and GPCR142 agonist. J Biol Chem 283: 23811–23818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugaard‐Kedstrom LM, Shabanpoor F, Hossain MA, Clark RJ, Ryan PJ, Craik DJ et al. (2011). Design, synthesis, and characterization of a single‐chain peptide antagonist for the relaxin‐3 receptor RXFP3. J Am Chem Soc 133: 4965–4974. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Pich EM, Baldwin HA, Rassnick S, Britton KT et al. (1994). Anti‐stress action of a corticotropin‐releasing factor antagonist on behavioral reactivity to stressors of varying type and intensity. Neuropsychopharmacology 11: 179–186. [DOI] [PubMed] [Google Scholar]
- Henniger MS, Ohl F, Holter SM, Weissenbacher P, Toschi N, Lorscher P et al. (2000). Unconditioned anxiety and social behaviour in two rat lines selectively bred for high and low anxiety‐related behaviour. Behav Brain Res 111: 153–163. [DOI] [PubMed] [Google Scholar]
- Hida T, Takahashi E, Shikata K, Hirohashi T, Sawai T, Seiki T et al. (2006). Chronic intracerebroventricular administration of relaxin‐3 increases body weight in rats. J Recept Signal Transduct Res 26: 147–158. [DOI] [PubMed] [Google Scholar]
- Higueruelo AP, Jubb H, Blundell TL (2013). Protein–protein interactions as druggable targets: recent technological advances. Curr Opin Pharmacol 13: 791–796. [DOI] [PubMed] [Google Scholar]
- Hojo K, Hossain MA, Tailhades J, Shabanpoor F, Wong LL, Ong‐Palsson EKE et al. (2016). Development of a single‐chain peptide agonist of the relaxin‐3 receptor using hydrocarbon stapling. J Med Chem. 59: 7445–7456. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Broberger C, Xu ZQ, Sergeyev V, Ubink R, Diez M (2000). Neuropeptides‐‐an overview. Neuropharmacology 39: 1337–1356. [DOI] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L et al. (2007). Oxytocin increases retention of social cognition in autism. Biol Psychiatry 61: 498–503. [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR et al. (2003). Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger's disorders. Neuropsychopharmacology 28: 193–198. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Mitchell BD, McMahon RP, Wonodi I, Buchanan RW et al. (2008). Sensory gating endophenotype based on its neural oscillatory pattern and heritability estimate. Arch Gen Psychiatry 65: 1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP (2007). Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212: 149–179. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Smith CM, Ryan PJ, Buchler E, Bathgate RA, Gundlach AL et al. (2013). Chemical synthesis and orexigenic activity of rat/mouse relaxin‐3. Amino Acids 44: 1529–1536. [DOI] [PubMed] [Google Scholar]
- Hostetler ED, Sanabria‐Bohorquez S, Fan H, Zeng Z, Gantert L, Williams M et al. (2011). Synthesis, characterization, and monkey positron emission tomography (PET) studies of [18F]Y1‐973, a PET tracer for the neuropeptide Y Y1 receptor. Neuroimage 54: 2635–2642. [DOI] [PubMed] [Google Scholar]
- Jayakody T, Marwari S, Lakshminarayanan R, Tan FC, Johannes CW, Dymock BW, Poulsen A, Herr DR, Dawe GS (2016). Hydrocarbon stapled B chain analogues of relaxin-3 retain biological activity. Peptides. doi: 10.1016/j.peptides.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Jimenez Vasquez PA, Salmi P, Ahlenius S, Mathe AA (2000). Neuropeptide Y in brains of the Flinders sensitive line rat, a model of depression. Effects of electroconvulsive stimuli and d‐amphetamine on peptide concentrations and locomotion. Behav Brain Res 111: 115–123. [DOI] [PubMed] [Google Scholar]
- Jubb H, Higueruelo AP, Winter A, Blundell TL (2012). Structural biology and drug discovery for protein–protein interactions. Trends Pharmacol Sci 33: 241–248. [DOI] [PubMed] [Google Scholar]
- Katz RJ (1982). Animal model of depression: pharmacological sensitivity of a hedonic deficit. Pharmacol Biochem Behav 16: 965–968. [DOI] [PubMed] [Google Scholar]
- Keck ME, Welt T, Muller MB, Uhr M, Ohl F, Wigger A et al. (2003). Reduction of hypothalamic vasopressinergic hyperdrive contributes to clinically relevant behavioral and neuroendocrine effects of chronic paroxetine treatment in a psychopathological rat model. Neuropsychopharmacology 28: 235–243. [DOI] [PubMed] [Google Scholar]
- Keck ME, Welt T, Wigger A, Renner U, Engelmann M, Holsboer F et al. (2001). The anxiolytic effect of the CRH(1) receptor antagonist R121919 depends on innate emotionality in rats. Eur J Neurosci 13: 373–380. [DOI] [PubMed] [Google Scholar]
- Keen‐Rhinehart E, Ondek K, Schneider JE (2013). Neuroendocrine regulation of appetitive ingestive behavior. Front Neurosci 7: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney GG, Kocsis B, Vertes RP (1995). Injections of muscimol into the median raphe nucleus produce hippocampal theta rhythm in the urethane anesthetized rat. Psychopharmacology (Berl) 120: 244–248. [DOI] [PubMed] [Google Scholar]
- Kohler S, Cierpinsky K, Kronenberg G, Adli M (2016). The serotonergic system in the neurobiology of depression: relevance for novel antidepressants. J Psychopharmacol 30: 13–22. [DOI] [PubMed] [Google Scholar]
- Kormos V, Gaszner B (2013). Role of neuropeptides in anxiety, stress, and depression: from animals to humans. Neuropeptides 47: 401–419. [DOI] [PubMed] [Google Scholar]
- Kuei C, Sutton S, Bonaventure P, Pudiak C, Shelton J, Zhu J et al. (2007). R3(BDelta23 27)R/I5 chimeric peptide, a selective antagonist for GPCR135 and GPCR142 over relaxin receptor LGR7: in vitro and in vivo characterization. J Biol Chem 282: 25425–25435. [DOI] [PubMed] [Google Scholar]
- Kumar JR, Rajkumar R, Farooq U, Lee LC, Tan FC, Dawe GS (2015). Evidence of D2 receptor expression in the nucleus incertus of the rat. Physiol Behav 151: 525–534. [DOI] [PubMed] [Google Scholar]
- Kumar JS, Walker M, Packiarajan M, Jubian V, Prabhakaran J, Chandrasena G et al. (2016). Radiosynthesis and in vivo evaluation of neuropeptide Y5 receptor (NPY5R) PET tracers. ACS Chem Neurosci 7: 540–545. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Chen FS, Pape HC, Heinrichs M (2013). Neuropeptide S receptor gene is associated with cortisol responses to social stress in humans. Biol Psychol 93: 304–307. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Wigger A (2002). High vs low anxiety‐related behavior rats: an animal model of extremes in trait anxiety. Behav Genet 32: 301–314. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Wigger A, Holsboer F, Neumann ID (1999). Hyper‐reactive hypothalamo‐pituitary‐adrenocortical axis in rats bred for high anxiety‐related behaviour. J Neuroendocrinol 11: 405–407. [DOI] [PubMed] [Google Scholar]
- Laroche S, Davis S, Jay TM (2000). Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus 10: 438–446. [DOI] [PubMed] [Google Scholar]
- Lawther AJ, Clissold ML, Ma S, Kent S, Lowry CA, Gundlach AL et al. (2015). Anxiogenic drug administration and elevated plus‐maze exposure in rats activate populations of relaxin‐3 neurons in the nucleus incertus and serotonergic neurons in the dorsal raphe nucleus. Neuroscience 303: 270–284. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Goodman WK, North WG, Chappell PB, Price LH, Pauls DL et al. (1994). The role of central oxytocin in obsessive compulsive disorder and related normal behavior. Psychoneuroendocrinology 19: 723–749. [DOI] [PubMed] [Google Scholar]
- Lee JH, Koh SQ, Guadagna S, Francis PT, Esiri MM, Chen CP et al. (2016). Altered relaxin family receptors RXFP1 and RXFP3 in the neocortex of depressed Alzheimer's disease patients. Psychopharmacology (Berl) 233: 591–598. [DOI] [PubMed] [Google Scholar]
- Lee LC, Rajkumar R, Dawe GS (2014). Selective lesioning of nucleus incertus with corticotropin releasing factor‐saporin conjugate. Brain Res 1543: 179–190. [DOI] [PubMed] [Google Scholar]
- Lenard NR, Berthoud HR (2008). Central and peripheral regulation of food intake and physical activity: pathways and genes. Obesity (Silver Spring) 16 (Suppl 3): S11–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenglos C, Mitra A, Guevremont G, Timofeeva E (2013). Sex differences in the effects of chronic stress and food restriction on body weight gain and brain expression of CRF and relaxin‐3 in rats. Genes Brain Behav 12: 370–387. [DOI] [PubMed] [Google Scholar]
- Lenglos C, Mitra A, Guevremont G, Timofeeva E (2014). Regulation of expression of relaxin‐3 and its receptor RXFP3 in the brain of diet‐induced obese rats. Neuropeptides 48: 119–132. [DOI] [PubMed] [Google Scholar]
- Lenglos C, Calvez J, Timofeeva E (2015). Sex-specific effects of relaxin-3 on food intake and brain expression of corticotropin-releasing factor in rats. Endocrinology 156: 523–533. [DOI] [PubMed] [Google Scholar]
- Liebsch G, Linthorst AC, Neumann ID, Reul JM, Holsboer F, Landgraf R (1998). Behavioral, physiological, and neuroendocrine stress responses and differential sensitivity to diazepam in two Wistar rat lines selectively bred for high‐ and low‐anxiety‐related behavior. Neuropsychopharmacology 19: 381–396. [DOI] [PubMed] [Google Scholar]
- Liu C, Chen J, Kuei C, Sutton S, Nepomuceno D, Bonaventure P et al. (2005). Relaxin‐3/insulin‐like peptide 5 chimeric peptide, a selective ligand for G protein‐coupled receptor (GPCR)135 and GPCR142 over leucine‐rich repeat‐containing G protein‐coupled receptor 7. Mol Pharmacol 67: 231–240. [DOI] [PubMed] [Google Scholar]
- Liu C, Kuei C, Sutton S, Shelton J, Zhu J, Nepomuceno D et al. (2009). Probing the functional domains of relaxin‐3 and the creation of a selective antagonist for RXFP3/GPCR135 over relaxin receptor RXFP1/LGR7. Ann N Y Acad Sci 1160: 31–37. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Pruessner J, Sousa N, Almeida OF, Van Dam AM, Rajkowska G et al. (2014). Neuropathology of stress. Acta Neuropathol 127: 109–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungu O, Anselmo K, Letourneau G, Mendrek A, Stip B, Lipp O et al. (2013). Neuronal correlates of appetite regulation in patients with schizophrenia: is there a basis for future appetite dysfunction? Eur Psychiatry 28: 293–301. [DOI] [PubMed] [Google Scholar]
- Ma et al. (in press).
- Ma S, Gundlach AL (2015). Ascending control of arousal and motivation: role of nucleus incertus and its peptide neuromodulators in behavioural responses to stress. J Neuroendocrinol 27: 457–467. [DOI] [PubMed] [Google Scholar]
- Ma S, Allocca G, Ong‐Palsson EK, Singleton CE, Hawkes D, McDougall SJ et al. (2016). Nucleus incertus promotes cortical desynchronization and behavioral arousal. Brain Struct Funct. doi: 10.1016/j.peptides.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Ma S, Blasiak A, Olucha‐Bordonau FE, Verberne AJ, Gundlach AL (2013). Heterogeneous responses of nucleus incertus neurons to corticotrophin‐releasing factor and coherent activity with hippocampal theta rhythm in the rat. J Physiol 591: 3981–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Bonaventure P, Ferraro T, Shen PJ, Burazin TC, Bathgate RA et al. (2007). Relaxin‐3 in GABA projection neurons of nucleus incertus suggests widespread influence on forebrain circuits via G‐protein‐coupled receptor‐135 in the rat. Neuroscience 144: 165–190. [DOI] [PubMed] [Google Scholar]
- Ma S, Olucha‐Bordonau FE, Hossain MA, Lin F, Kuei C, Liu C et al. (2009). Modulation of hippocampal theta oscillations and spatial memory by relaxin‐3 neurons of the nucleus incertus. Learn Mem 16: 730–742. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000). Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20: 9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maletic V, Robinson M, Oakes T, Iyengar S, Ball SG, Russell J (2007). Neurobiology of depression: an integrated view of key findings. Int J Clin Pract 61: 2030–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Chiamulera C, Geyer MA, Tricklebank M, Steckler T (2009). Removing obstacles in neuroscience drug discovery: the future path for animal models. Neuropsychopharmacology 34: 74–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Bellver S, Cervera‐Ferri A, Martinez‐Ricos J, Ruiz‐Torner A, Luque‐Garcia A, Blasco‐Serra A et al. (2015). Regular theta‐firing neurons in the nucleus incertus during sustained hippocampal activation. Eur J Neurosci 41: 1049–1067. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, McDonald CH, Brooks PJ, Goldman D (1996). An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol Behav 60: 1209–1215. [DOI] [PubMed] [Google Scholar]
- McGowan BM, Minnion JS, Murphy KG, Roy D, Stanley SA, Dhillo WS et al. (2014). Relaxin‐3 stimulates the neuro‐endocrine stress axis via corticotrophin‐releasing hormone. J Endocrinol 221: 337–346. [DOI] [PubMed] [Google Scholar]
- McGowan BM, Stanley SA, Donovan J, Thompson EL, Patterson M, Semjonous NM et al. (2008). Relaxin‐3 stimulates the hypothalamic‐pituitary‐gonadal axis. Am J Physiol Endocrinol Metab 295: E278–E286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan BM, Stanley SA, Smith KL, Minnion JS, Donovan J, Thompson EL et al. (2006). Effects of acute and chronic relaxin‐3 on food intake and energy expenditure in rats. Regul Pept 136: 72–77. [DOI] [PubMed] [Google Scholar]
- McGowan BM, Stanley SA, Smith KL, White NE, Connolly MM, Thompson EL et al. (2005). Central relaxin‐3 administration causes hyperphagia in male Wistar rats. Endocrinology 146: 3295–3300. [DOI] [PubMed] [Google Scholar]
- McGowan BM, Stanley SA, White NE, Spangeus A, Patterson M, Thompson EL et al. (2007). Hypothalamic mapping of orexigenic action and Fos‐like immunoreactivity following relaxin‐3 administration in male Wistar rats. Am J Physiol Endocrinol Metab 292: E913–E919. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ (2004). A two‐dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav Rev 28: 285–305. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Gray JA (2000). Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. J Affect Disord 61: 161–176. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Swart C, Neo P, Bates V, Glue P (2013). Anti‐anxiety drugs reduce conflict‐specific “theta”‐‐a possible human anxiety‐specific biomarker. J Affect Disord 148: 104–111. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Watanabe Y, Tanaka M (2008). Developmental expression and serotonergic regulation of relaxin 3/INSL7 in the nucleus incertus of rat brain. Regul Pept 145: 54–59. [DOI] [PubMed] [Google Scholar]
- Munro J, Skrobot O, Sanyoura M, Kay V, Susce MT, Glaser PE et al. (2012). Relaxin polymorphisms associated with metabolic disturbance in patients treated with antipsychotics. J Psychopharmacol 26: 374–379. [DOI] [PubMed] [Google Scholar]
- Musazzi L, Marrocco J (2016). Stress response and perinatal reprogramming: unraveling (mal)adaptive strategies. Neural Plast 2016 .6752193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa CM, Shikata K, Uesugi M, Katayama H, Aoshima K, Tahara K et al. (2013). Prediction of relaxin‐3‐induced downstream pathway resulting in anxiolytic‐like behaviors in rats based on a microarray and peptidome analysis. J Recept Signal Transduct Res 33: 224–233. [DOI] [PubMed] [Google Scholar]
- Nategh M, Nikseresht S, Khodagholi F, Motamedi F (2016). Inactivation of nucleus incertus impairs passive avoidance learning and long term potentiation of the population spike in the perforant path‐dentate gyrus evoked field potentials in rats. Neurobiol Learn Mem 130: 185–193. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE (2010). Animal models of neuropsychiatric disorders. Nat Neurosci 13: 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM (2002). Neurobiology of depression. Neuron 34: 13–25. [DOI] [PubMed] [Google Scholar]
- Newcomer JW (2007). Metabolic considerations in the use of antipsychotic medications: a review of recent evidence. J Clin Psychiatry 68 (Suppl 1): 20–27. [PubMed] [Google Scholar]
- Notarianni E (2013). Hypercortisolemia and glucocorticoid receptor‐signaling insufficiency in Alzheimer's disease initiation and development. Curr Alzheimer Res 10: 714–731. [DOI] [PubMed] [Google Scholar]
- Nunez A, Cervera‐Ferri A, Olucha‐Bordonau F, Ruiz‐Torner A, Teruel V (2006). Nucleus incertus contribution to hippocampal theta rhythm generation. Eur J Neurosci 23: 2731–2738. [DOI] [PubMed] [Google Scholar]
- Nunez A, de Andres I, Garcia‐Austt E (1991). Relationships of nucleus reticularis pontis oralis neuronal discharge with sensory and carbachol evoked hippocampal theta rhythm. Exp Brain Res 87: 303–308. [DOI] [PubMed] [Google Scholar]
- Nutt DJ (2006). The role of dopamine and norepinephrine in depression and antidepressant treatment. J Clin Psychiatry 67 (Suppl 6): 3–8. [PubMed] [Google Scholar]
- Oddie SD, Bland BH, Colom LV, Vertes RP (1994). The midline posterior hypothalamic region comprises a critical part of the ascending brainstem hippocampal synchronizing pathway. Hippocampus 4: 454–473. [DOI] [PubMed] [Google Scholar]
- Ohl F, Toschi N, Wigger A, Henniger MS, Landgraf R (2001). Dimensions of emotionality in a rat model of innate anxiety. Behav Neurosci 115: 429–436. [PubMed] [Google Scholar]
- Olucha‐Bordonau FE, Teruel V, Barcia‐Gonzalez J, Ruiz‐Torner A, Valverde‐Navarro AA, Martinez‐Soriano F (2003). Cytoarchitecture and efferent projections of the nucleus incertus of the rat. J Comp Neurol 464: 62–97. [DOI] [PubMed] [Google Scholar]
- Overstreet DH (1993). The Flinders sensitive line rats: a genetic animal model of depression. Neurosci Biobehav Rev 17: 51–68. [DOI] [PubMed] [Google Scholar]
- Overstreet DH (2012). Modeling depression in animal models. Methods Mol Biol 829: 125–144. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Pucilowski O, Rezvani AH, Janowsky DS (1995). Administration of antidepressants, diazepam and psychomotor stimulants further confirms the utility of Flinders sensitive line rats as an animal model of depression. Psychopharmacology (Berl) 121: 27–37. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Rezvani AH, Djouma E, Parsian A, Lawrence AJ (2007). Depressive‐like behavior and high alcohol drinking co‐occur in the FH/WJD rat but appear to be under independent genetic control. Neurosci Biobehav Rev 31: 103–114. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL (2008). The HPA axis in major depression: classical theories and new developments. Trends Neurosci 31: 464–468. [DOI] [PubMed] [Google Scholar]
- Passerin AM, Cano G, Rabin BS, Delano BA, Napier JL, Sved AF (2000). Role of locus coeruleus in foot shock‐evoked Fos expression in rat brain. Neuroscience 101: 1071–1082. [DOI] [PubMed] [Google Scholar]
- Patil et al. (in press).
- Pereira CW, Santos FN, Sanchez‐Perez AM, Otero‐Garcia M, Marchioro M, Ma S et al. (2013). Electrolytic lesion of the nucleus incertus retards extinction of auditory conditioned fear. Behav Brain Res 247: 201–210. [DOI] [PubMed] [Google Scholar]
- Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K et al. (1994). Distribution of corticotropin‐releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci U S A 91: 8777–8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pull CB (2007). Combined pharmacotherapy and cognitive‐behavioural therapy for anxiety disorders. Curr Opin Psychiatry 20: 30–35. [DOI] [PubMed] [Google Scholar]
- Rajkumar R, See LK, Dawe GS (2013). Acute antipsychotic treatments induce distinct c‐Fos expression patterns in appetite‐related neuronal structures of the rat brain. Brain Res 1508: 34–43. [DOI] [PubMed] [Google Scholar]
- Rajkumar R, Wu Y, Farooq U, Tan WH, Dawe GS (2016). Stress activates the nucleus incertus and modulates plasticity in the hippocampo‐medial prefrontal cortical pathway. Brain Res Bull 120: 83–89. [DOI] [PubMed] [Google Scholar]
- Ramirez MJ, Lai MK, Tordera RM, Francis PT (2014). Serotonergic therapies for cognitive symptoms in Alzheimer's disease: rationale and current status. Drugs 74: 729–736. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K et al. (2011). Post‐traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470: 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Parsian A, Overstreet DH (2002). The fawn‐hooded (FH/Wjd) rat: a genetic animal model of comorbid depression and alcoholism. Psychiatr Genet 12: 1–16. [DOI] [PubMed] [Google Scholar]
- Rivest S, Laflamme N, Nappi RE (1995). Immune challenge and immobilization stress induce transcription of the gene encoding the CRF receptor in selective nuclei of the rat hypothalamus. J Neurosci 15: 2680–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL (2016). DREADDs for neuroscientists. Neuron 89: 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MK, Bingham B, Shah A, Joshi A, Frazer A, Strong R et al. (2012). Effects of chronic plus acute prolonged stress on measures of coping style, anxiety, and evoked HPA‐axis reactivity. Neuropharmacology 63: 1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotzinger S, Lovejoy DA, Tan LA (2010). Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides 31: 736–756. [DOI] [PubMed] [Google Scholar]
- Ryan PJ, Buchler E, Shabanpoor F, Hossain MA, Wade JD, Lawrence AJ et al. (2013a). Central relaxin‐3 receptor (RXFP3) activation decreases anxiety‐ and depressive‐like behaviours in the rat. Behav Brain Res 244: 142–151. [DOI] [PubMed] [Google Scholar]
- Ryan PJ, Kastman HE, Krstew EV, Rosengren KJ, Hossain MA, Churilov L et al. (2013b). Relaxin‐3/RXFP3 system regulates alcohol‐seeking. Proc Natl Acad Sci U S A 110: 20789–20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PJ, Ma S, Olucha‐Bordonau FE, Gundlach AL (2011). Nucleus incertus‐an emerging modulatory role in arousal, stress and memory. Neurosci Biobehav Rev 35: 1326–1341. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Twardowska K (1999). The dexamethasone/corticotropin‐releasing hormone test in depression in bipolar and unipolar affective illness. J Psychiatr Res 33: 363–370. [DOI] [PubMed] [Google Scholar]
- Sabban EL, Alaluf LG, Serova LI (2016). Potential of neuropeptide Y for preventing or treating post‐traumatic stress disorder. Neuropeptides 56: 19–24. [DOI] [PubMed] [Google Scholar]
- Sah R, Ekhator NN, Strawn JR, Sallee FR, Baker DG, Horn PS et al. (2009). Low cerebrospinal fluid neuropeptide Y concentrations in posttraumatic stress disorder. Biol Psychiatry 66: 705–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S et al. (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805–809. [DOI] [PubMed] [Google Scholar]
- Santos FN, Pereira CW, Sanchez‐Perez AM, Otero‐Garcia M, Ma S, Gundlach AL et al. (2016). Comparative distribution of relaxin‐3 inputs and calcium‐binding protein‐positive neurons in rat amygdala. Front Neuroanat 10: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JB, Drevets WC (2013). Neuroreceptor imaging in depression. Neurobiol Dis 52: 49–65. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG (1995). Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav Neurosci 109: 1063–1073. [DOI] [PubMed] [Google Scholar]
- Senba E, Matsunaga K, Tohyama M, Noguchi K (1993). Stress‐induced c‐fos expression in the rat brain: activation mechanism of sympathetic pathway. Brain Res Bull 31: 329–344. [DOI] [PubMed] [Google Scholar]
- Serova LI, Tillinger A, Alaluf LG, Laukova M, Keegan K, Sabban EL (2013). Single intranasal neuropeptide Y infusion attenuates development of PTSD‐like symptoms to traumatic stress in rats. Neuroscience 236: 298–312. [DOI] [PubMed] [Google Scholar]
- Shabanpoor F, Akhter Hossain M, Ryan PJ, Belgi A, Layfield S, Kocan M et al. (2012). Minimization of human relaxin‐3 leading to high‐affinity analogues with increased selectivity for relaxin‐family peptide 3 receptor (RXFP3) over RXFP1. J Med Chem 55: 1671–1681. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID (2010). Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology 58: 56–61. [DOI] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA (2011). A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 12: 585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CM, Lawrence AJ, Sutton SW, Gundlach AL (2009). Behavioral phenotyping of mixed background (129S5:B6) relaxin‐3 knockout mice. Ann N Y Acad Sci 1160: 236–241. [DOI] [PubMed] [Google Scholar]
- Smith CM, Ryan PJ, Hosken IT, Ma S, Gundlach AL (2011). Relaxin‐3 systems in the brain‐the first 10 years. J Chem Neuroanat 42: 262–275. [DOI] [PubMed] [Google Scholar]
- Smith CM, Walker AW, Hosken IT, Chua BE, Zhang C, Haidar M et al. (2014). Relaxin‐3/RXFP3 networks: an emerging target for the treatment of depression and other neuropsychiatric diseases? Front Pharmacol 5: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al. (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Almli LM, Fani N, Gutman DA, Bradley B, Norrholm SD et al. (2014). PACAP receptor gene polymorphism impacts fear responses in the amygdala and hippocampus. Proc Natl Acad Sci U S A 111: 3158–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton SW, Bonaventure P, Kuei C, Roland B, Chen J, Nepomuceno D et al. (2004). Distribution of G‐protein‐coupled receptor (GPCR)135 binding sites and receptor mRNA in the rat brain suggests a role for relaxin‐3 in neuroendocrine and sensory processing. Neuroendocrinology 80: 298–307. [DOI] [PubMed] [Google Scholar]
- Sutton SW, Shelton J, Smith C, Williams J, Yun S, Motley T et al. (2009). Metabolic and neuroendocrine responses to RXFP3 modulation in the central nervous system. Ann N Y Acad Sci 1160: 242–249. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Iijima N, Miyamoto Y, Fukusumi S, Itoh Y, Ozawa H et al. (2005). Neurons expressing relaxin 3/INSL 7 in the nucleus incertus respond to stress. Eur J Neurosci 21: 1659–1670. [DOI] [PubMed] [Google Scholar]
- Timofeeva E, Huang Q, Richard D (2003). Effects of treadmill running on brain activation and the corticotropin‐releasing hormone system. Neuroendocrinology 77: 388–405. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W (2012). Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large‐scale networks. Neuron 75: 963–980. [DOI] [PubMed] [Google Scholar]
- Valjakka A, Vartiainen J, Tuomisto L, Tuomisto JT, Olkkonen H, Airaksinen MM (1998). The fasciculus retroflexus controls the integrity of REM sleep by supporting the generation of hippocampal theta rhythm and rapid eye movements in rats. Brain Res Bull 47: 171–184. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH (1969). Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol 26: 407–418. [DOI] [PubMed] [Google Scholar]
- Verdine GL, Walensky LD (2007). The challenge of drugging undruggable targets in cancer: lessons learned from targeting BCL‐2 family members. Clin Cancer Res 13: 7264–7270. [DOI] [PubMed] [Google Scholar]
- Vertes RP (2005). Hippocampal theta rhythm: a tag for short‐term memory. Hippocampus 15: 923–935. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B (1997). Brainstem‐diencephalo‐septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience 81: 893–926. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Martin GF (1988). Autoradiographic analysis of ascending projections from the pontine and mesencephalic reticular formation and the median raphe nucleus in the rat. J Comp Neurol 275: 511–541. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Viana Di Prisco G (2004). Theta rhythm of the hippocampus: subcortical control and functional significance. Behav Cogn Neurosci Rev 3: 173–200. [DOI] [PubMed] [Google Scholar]
- Viana Di Prisco G, Albo Z, Vertes RP, Kocsis B (2002). Discharge properties of neurons of the median raphe nucleus during hippocampal theta rhythm in the rat. Exp Brain Res 145: 383–394. [DOI] [PubMed] [Google Scholar]
- Viero C, Shibuya I, Kitamura N, Verkhratsky A, Fujihara H, Katoh A et al. (2010). Review: oxytocin: crossing the bridge between basic science and pharmacotherapy. CNS Neurosci Ther 16: e138–e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova OS (1995). Expression, control, and probable functional significance of the neuronal theta‐rhythm. Prog Neurobiol 45: 523–583. [DOI] [PubMed] [Google Scholar]
- Walensky LD, Bird GH (2014). Hydrocarbon‐stapled peptides: principles, practice, and progress. J Med Chem 57: 6275–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Miyamoto Y, Matsuda T, Tanaka M (2011a). Relaxin‐3/INSL7 regulates the stress‐response system in the rat hypothalamus. J Mol Neurosci 43: 169–174. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Tsujimura A, Takao K, Nishi K, Ito Y, Yasuhara Y et al. (2011b). Relaxin‐3‐deficient mice showed slight alteration in anxiety‐related behavior. Front Behav Neurosci 5: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Vanderwolf CH (1973). Hippocampal EEG and behavior: changes in amplitude and frequency of RSA (theta rhythm) associated with spontaneous and learned movement patterns in rats and cats. Behav Biol 8: 461–484. [DOI] [PubMed] [Google Scholar]
- Widdowson PS, Ordway GA, Halaris AE (1992). Reduced neuropeptide Y concentrations in suicide brain. J Neurochem 59: 73–80. [DOI] [PubMed] [Google Scholar]
- Widerlov E, Lindstrom LH, Wahlestedt C, Ekman R (1988). Neuropeptide Y and peptide YY as possible cerebrospinal fluid markers for major depression and schizophrenia, respectively. J Psychiatr Res 22: 69–79. [DOI] [PubMed] [Google Scholar]
- Wigger A, Loerscher P, Weissenbacher P, Holsboer F, Landgraf R (2001). Cross‐fostering and cross‐breeding of HAB and LAB rats: a genetic rat model of anxiety. Behav Genet 31: 371–382. [DOI] [PubMed] [Google Scholar]
- Wigger A, Sanchez MM, Mathys KC, Ebner K, Frank E, Liu D et al. (2004). Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: critical role of vasopressin. Neuropsychopharmacology 29: 1–14. [DOI] [PubMed] [Google Scholar]
- Willner P (2005). Chronic mild stress (CMS) revisited: consistency and behavioural‐neurobiological concordance in the effects of CMS. Neuropsychobiology 52: 90–110. [DOI] [PubMed] [Google Scholar]
- Zhang C, Chua BE, Yang A, Shabanpoor F, Hossain MA, Wade JD et al. (2015). Central relaxin‐3 receptor (RXFP3) activation reduces elevated, but not basal, anxiety‐like behaviour in C57BL/6 J mice. Behav Brain Res 292: 125–132. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhu M, Zhao M, Chen W, Fu Y, Liu Y et al. (2013). The plasma levels of relaxin‐2 and relaxin‐3 in patients with diabetes. Clin Biochem 46: 1713–1716. [DOI] [PubMed] [Google Scholar]


