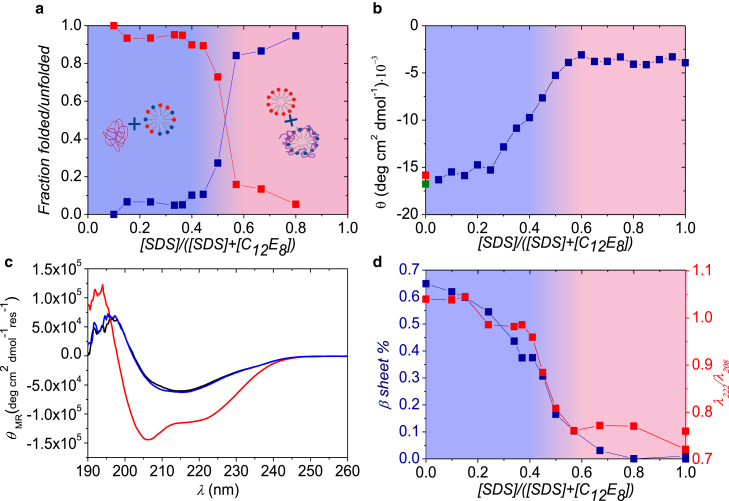
Figure 2.
Results for βLG and SDS samples mixed with C12E8. (a) Part of the protein that is either folded (upper data points at low mole fractions) or unfolded around SDS micelles (lower data points at low mole fractions) is shown. (b) Near-UV CD data at 293 nm of βLG in micelles (data as a function of mole fraction) buffer (upper point at zero mole fraction), and in 25 mM C12E8 (lower point at zero mole fraction) are shown. (c) Shown here are CD spectra for pure βLG (black), βLG mixed with SDS and C12E8 at SDS mole fractions of 0.8 (curve with deepest minimum) and 0.37 (nearly coinciding with black curve), respectively. (d) The fraction of β-sheet (points going to zero at a mole fraction of 0.8), and λ222/λ208 ratio (upper points at high mole fractions) is given. Background shading in (a), (b), and (d) indicates whether the protein is mainly free in solution (right part) or bound to micelles (left part). To see this figure in color, go online.