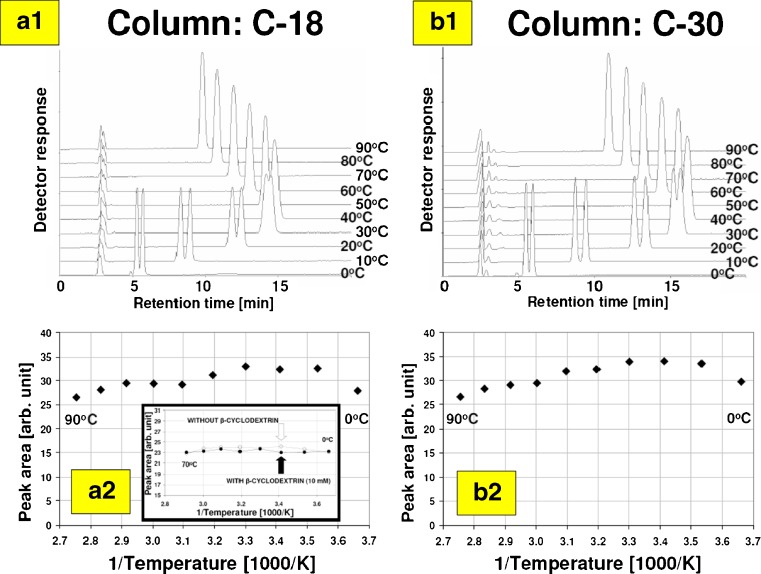
Fig. 3.
Separation of acenaphthenol enantiomers under HPLC conditions using β-CD additive to binary mobile phase composed of acetonitrile:water (35:65, v/v) using C-18 (a1) and C-30 (b1) stationary phases (both 15 cm long columns; mobile phase flow 0.5 mL/min) at different temperatures and corresponding peak integration results (a2, b2; peak areas for separated enantiomers were summarized). Graph inserted within plot a2 refers to acenaphthenol peak area data obtained on Supelcosil LC-18 column (10 cm, flow 1 mL/min) without and with β-cyclodextrin additive (labeled as empty circles and black dots, respectively)