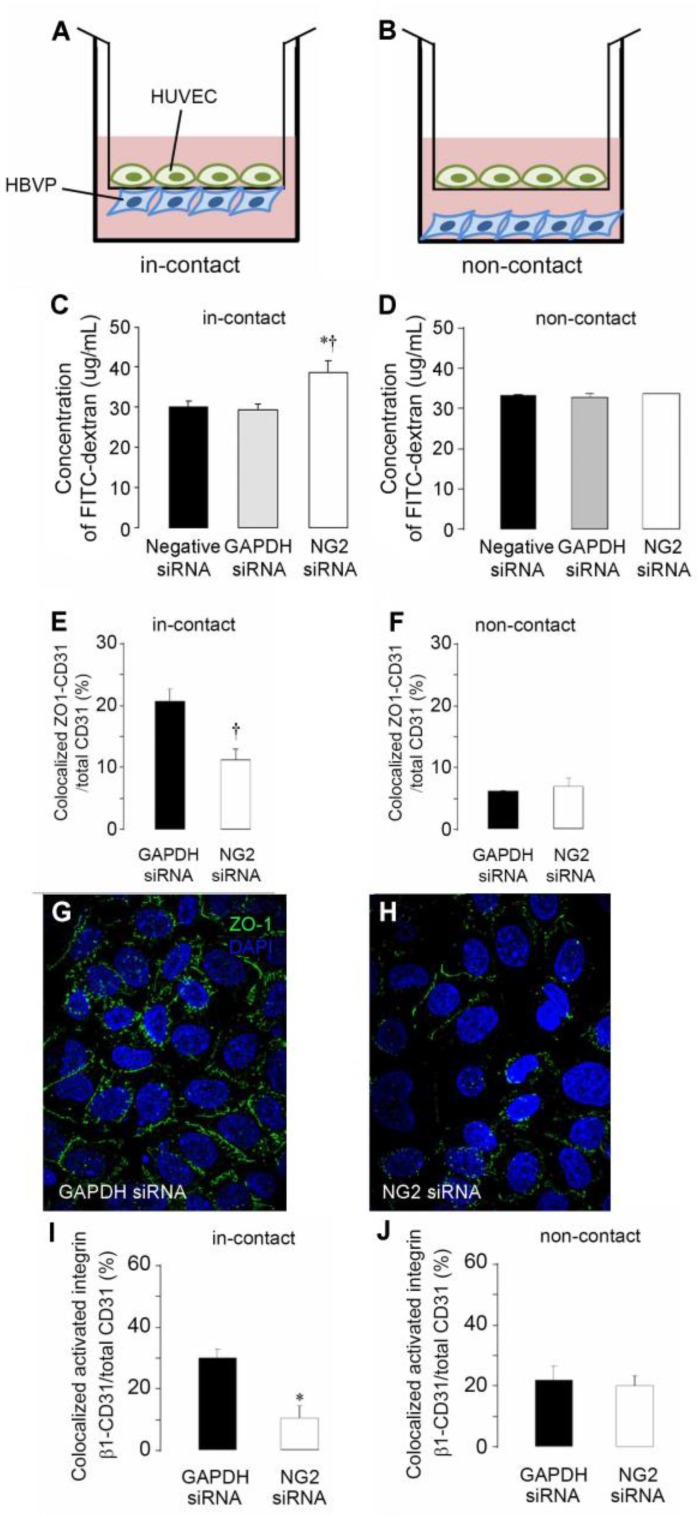
Figure 6.
NG2 knockdown in pericytes reduces integrin activation and formation of endothelial junctions. Pericyte-endothelial cell interactions were investigated using in-contact (A) and non-contact (B) Transwell co-cultures. HUVEC: human umbilical vein endothelial cells. HBVP: human brain vascular pericytes. NG2 knockdown in pericytes reveals that NG2 expression is required for enhanced barrier function (reduced leakage of FITC-dextran from upper to lower chamber) by the endothelial monolayer in the in-contact model (C) but not in the non-contact model (D). GAPDH siRNA and negative (scrambled NG2) siRNA serve as controls for NG2 siRNA in these experiments. In the in-contact model (E), NG2 expression by pericytes is needed for formation of the ZO-1 positive endothelial junctions that underlie barrier function. Expression of NG2 by pericytes does not affect endothelial junction formation in the non-contact model (F). Junctions are quantified as percentage of CD31-positive cells with ZO-1 positive junctions. Robust formation of ZO-1 positive endothelial junctions (green) is observed when NG2-positive pericytes are used in the in-contact model (G: blue = DAPI). Endothelial junction formation is impaired in the in-contact model when NG2 is knocked down in pericytes (H). In the in-contact model, β1 integrin activation in endothelial cells is reduced by knockdown of NG2 in pericytes (I). In the non-contact model, endothelial β1 integrin activation is not affected by NG2 knockdown in pericytes (J). The HUTS-21 antibody was used to quantify β1 integrin activation in HUVECs. Activation is quantified as the percentage of CD31-positive cells with HUTS-21 positive junctions. Data taken from [9].