Fig. 4.
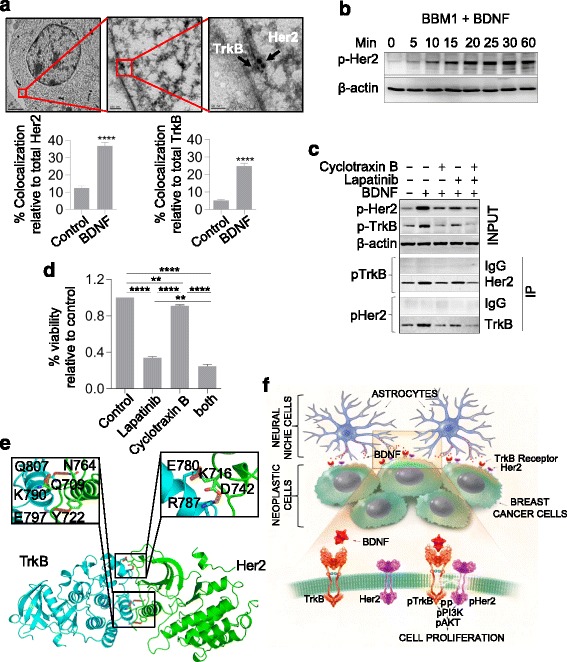
Tropomyosin-related kinase B (TrkB) and human epidermal growth factor receptor 2 (Her2) heterodimerize and activate upon brain-derived neurotrophic factor (BDNF) administration. a Representative post-embedding electron microscopy image (top) of TrkB and Her2 clustering at the cell membrane upon BDNF treatment; t = 30 minutes, TrkB = 10 nm, Her2 = 20 nm. Quantification (bottom) of co-localized Her2 or TrkB receptors relative to total Her2 or TrkB receptors, respectively (bars indicate SEM). b Western blot analysis of Her2 activated by exogenous BDNF over 1 h in BBM1 cells. c Co-immunoprecipitation of TrkB and Her2 from BBM1 cells grown in the presence of lapatinib (50 μM) and/or cyclotraxin B (20 μM). Her2 and TrkB immunoprecipitations were analyzed by western blotting with anti-TrkB and anti-Her2 antibodies, respectively. d Molecular model of TrkB (left) and Her2 (right). Insets indicate amino acids on TrkB and Her2 involved in possible hydrogen bonding. e Quantification of viable BBM1 cells grown in the presence of Her2 and TrkB inhibitors lapatinib (50 μM) and cyclotraxin B (20 μM), respectively. f Predicted model of paracrine signaling between breast cancer cells and the brain microenvironment. Her2 receptor (orange) and TrkB receptor (purple) on the cell surface membrane (green) are phosphorylated upon binding of BDNF (red) secreted by surrounding astrocytes (blue)
