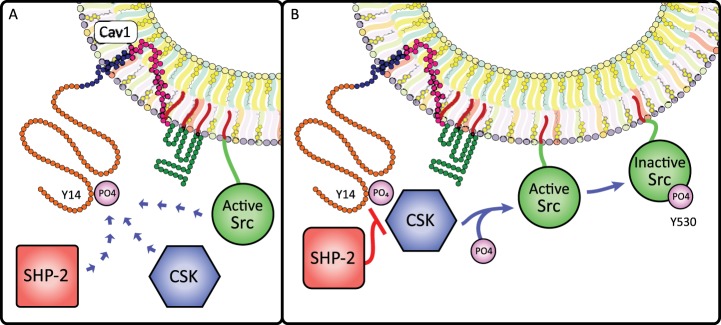
Fig. 6.
Cav1 phosphorylation influences Src signaling. A: Cav1Y14 phosphorylation creates a Src homology 2 (SH2)-binding site that regulates Src activity through binding of Src and recruitment of two cytosolic Src-regulating enzymes: COOH-terminal Src kinase (CSK) and SH2 domain-containing non-transmembrane protein tyrosine phosphatase (SHP-2). B: when recruited to caveolae by pCav1Y14, CSK deactivates Src by phosphorylating SrcY530; however, pCav1Y14 binding to SHP-2 blocks CSK recruitment and prevents SrcY530 phosphorylation, increasing the duration of Src activity. Thus, Cav1 phosphorylation permits a rheostat-like control of Src signaling.