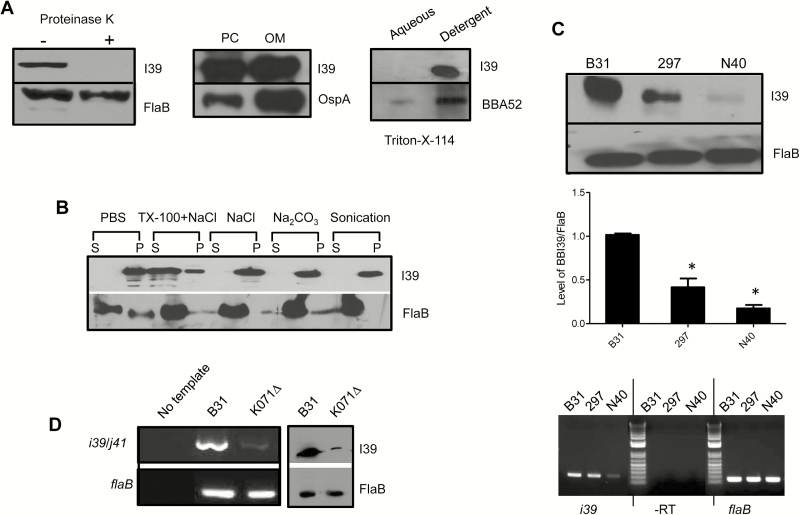
Figure 2.
BBI39 encodes for an outer membrane surface-exposed protein. A, BBI39 is exposed on the spirochete surface. Borrelia burgdorferi cells were treated with (+) or without (−) proteinase K and processed for immunoblot analysis using either BBI39 or subsurface (FlaB) protein antibodies (left panel). BBI39 is distributed in the outer membrane of spirochetes. Borrelia burgdorferi lysates were separated into protoplasmic cylinder (PC) and outer membrane (OM) fraction and assessed by immunoblotting (middle panel). BBI39 is partitioned in the detergent phases of B. burgdorferi proteins. Spirochete lysates were subjected to Triton X-114 phase partitioning and immunoblotted (right panel). B, Association of BBI39 with borrelial membranes. Borrelia burgdorferi lysates were treated with salt and detergent, and supernatant (S) and pellet (P) fractions were assessed by immunoblotting. C, Production of BBI39 in representative infectious isolates of B. burgdorferi. Different isolates of B. burgdorferi were grown to an equal cell density (108 cells/mL). Spirochete lysates were prepared and immunoblotted with BBI39 antibody generated against B. burgdorferi B31 isolate (upper panel) and subjected to quantitative densitometry (middle panel). The levels of BBI39 are significantly reduced in 297 and N40 strains (*P < .05). The bars represent the mean values from 3 independent experiments, and the error bars represent the SEM. The lower panel shows reverse transcription-polymerase chain reaction (RT-PCR) analysis of bbi39 transcripts. Levels of flaB served as loading control, and absence of DNA contamination was ensured by RT-PCR reaction in the absence of reverse transcriptase (-RT). D, Assessment of relative transcript abundance of bbi39 and its paralogs. The mRNA levels of bbi39 or its paralogous genes in B. burgdorferi and an isogenic B31 isolate that is missing lp28 (hence missing bbi36, bbi38, and bbi39), designated K071∆, were assessed by RT-PCR. The primer information is presented in Supplementary Table 1.