Abstract
Cardiorenal syndrome type 1 causes acute kidney injury but is poorly understood; animal models and diagnostic aids are lacking. Robust noninvasive measurements of glomerular filtration rate are required for injury models and clinical use. Several have been described but are untested in translational models and suffer from biologic interference. We developed a mouse model of cardiorenal syndrome and tested the novel near-infrared fluorophore ZW800-1 to assess renal and cardiac function. We performed murine cardiac arrest and cardiopulmonary resuscitation followed by transthoracic echocardiography, 2 and 24 h later. Transcutaneous fluorescence of ZW800-1 bolus dispersion and clearance was assessed with whole animal imaging and compared with glomerular filtration rate (GFR; inulin clearance), tubular cell death (using unbiased stereology), and serum creatinine. Correlation, Bland-Altman, and polar analyses were used to compare GFR with ZW800-1 clearance. Cardiac arrest and cardiopulmonary resuscitation caused reversible cardiac failure, halving fractional shortening of the left ventricle (n = 12, P = 0.03). Acute kidney injury resulted with near-zero GFR and sixfold increase in serum creatinine 24 h later (n = 16, P < 0.01). ZW800-1 biodistribution and clearance were exclusively renal. ZW800-1 t1/2 and clearance correlated with GFR (r = 0.92, n = 31, P < 0.0001). ZW800-1 fluorescence was reduced in cardiac arrest, and cardiopulmonary resuscitation-treated mice compared with sham animals 810 s after injection (P < 0.01) and bolus time-dispersion curves demonstrated that ZW800-1 fluorescence dispersion correlated with left ventricular function (r = 0.74, P < 0.01). Cardiac arrest and cardiopulmonary resuscitation lead to experimental cardiorenal syndrome type 1. ZW800-1, a small near-infrared fluorophore being developed for clinical intraoperative imaging, is favorable for evaluating cardiac and renal function noninvasively.
glomerular filtration rate (GFR) is a critical measure of renal function and dysfunction. Reduction of GFR characterizes much renal pathophysiology and is a critical diagnostic criterion for acute and chronic kidney disease. However, measurement of GFR in vivo and in the clinical setting remains challenging, leading to undesirable reliance on surrogate markers (25). Barriers to measurement of GFR include invasiveness, particularly the need for multiple measurements of serum indicator concentration, and potential off-target effects of the pharmacologic indicator, entailing regulatory approval and expense. In recent years, investigators have developed noninvasive methods of measuring GFR using radioactive isotope decay or fluorescence decay, which can be detected transcutaneously (2, 29, 31, 34). Non- or minimally invasive transcutaneous fluorimetric methods based on fluorescein isothiocyanate (FITC) conjugated to polysaccharides, which are known GFR indicators, have demonstrated feasibility, reliability, and safety in small animals (33, 35).
Near-infrared fluorophores offer advantages over visible-range fluorophores, including reduced signal attenuation due to biologic absorbance, as few biologic compounds absorb in the near-infrared. As such, near-infrared fluorophores are being widely developed for medical applications, such as intraoperative wayfinding. Because of minimal tissue attenuation, near-infrared fluorophores are especially useful in transcutaneous applications. ZW800-1 is a novel near-infrared fluorophore being developed for intraoperative cancer visualization. It is nontoxic, and early studies suggested it is completely renally cleared (10). Zwitterions with neutral overall charge exhibit reduced lipophilicity and interact minimally with proteins due to charge shielding (11). These characteristics are salutary for transcutaneous measurement of GFR, but no data yet compare ZW800-1 against gold-standard measures of GFR in animals with normal and abnormal kidney function.
Acute kidney injury (AKI) is a morbid complication of critical illness and surgery. Cardiorenal syndrome type 1 (CRS1) is the cause of 25% of all AKI; it quintuples 28-day mortality in patients with cardiovascular injury and exhibits persistent effect, doubling long-term mortality (37). Animal models of CRS1 are lacking, as are diagnostic tools. We have previously used cardiac arrest and cardiopulmonary resuscitation (CA/CPR) to model ischemic AKI. As the cause of ischemia in this model is cardiac arrest, we hypothesized that mice subjected to CA/CPR develop experimental CRS1 and that transcutaneous measurement of GFR using ZW800-1 and near-infrared imaging was feasible in this model. During our transcutaneous fluorescence imaging studies, we serendipitously noted that the postinjection distribution of ZW800-1 fluorescence was prolonged in mice subjected to CA/CPR compared with sham. Therefore, we undertook investigation of a secondary hypothesis, that ZW800-1 fluorescence distribution rate is associated with the extent of cardiac dysfunction after CA/CPR.
MATERIALS AND METHODS
CA/CPR.
Animal experiments were approved by the Institutional Animal Care and Use Committee of Oregon Health & Science University or Harvard Medical School and were conducted in accordance with the National Institutes of Health's “Guide for the Care and Use of Laboratory Animals.” CA/CPR and sham procedure were performed as described previously (17–20, 22). Briefly, under isoflurane general anesthesia, mice (C57BL/6, male and female, 10–47 wk old, n = 31) were orotracheally intubated with a 22-gauge catheter and a polyethylene (PE)-10 catheter was placed in the jugular vein. Transcutaneous needle electrodes were placed to monitor the electrocardiogram. Normothermia (36.5–37.5°C was maintained using a rectal temperature monitor, heating lamp, temperature controller, and insulating blanket. Cardiac arrest was induced with intravenous potassium chloride. Ten minutes after cardiac arrest (or 8 min in TTE and bolus dispersion studies), chest compressions (300/min), and administration of intravenous epinephrine (8–16 µg) were initiated. Resuscitation was stopped when return of spontaneous circulation was not observed within 180 s. After resumption of satisfactory respiratory effort, the trachea was extubated, the jugular cannula was removed, and the mice were moved to a recovery cage and kept on a 37°C warming pad.
Echocardiography and measurement of left and right ventricular cardiac function.
Mice underwent transthoracic echocardiography 24 h after sham procedure and 2 h or 24 h after after CA/CPR. A 55-mHz phased array probe was used to image the left ventricle (LV) in a short-axis midpapillary view and the right ventricular outflow tract (RVOT) and proximal pulmonary artery (PA) in a basal short axis view. LV fractional area change, fractional shortening, ejection fraction, and PA ejection time and acceleration time were measured using two-dimensional and Doppler imaging. As a midsystolic notch in the PA Doppler tracing has been described as an indicator of right ventricle (RV) dysfunction (27), we assessed each recorded systolic PA tracing for the presence of this finding.
ZW800-1 bolus biodistribution assessment.
Administration of 10 nmol (0.2 mg/kg) ZW800-1 was made to CD-1 mice (n = 3) by bolus intravenous injection. Four hours later, laparotomy was performed under general anesthesia and visible range and 800-nm intraoperative imaging of the abdomen was performed. Following death, individual organs (heart, lung, liver, pancreas/spleen, kidneys, duodenum, large intestine, and skeletal muscle) were carefully removed and imaged in the visible range and at 800 nm. Results were expressed as signal/background (defined as skeletal muscle fluorescence).
Measurement of glomerular filtration rate and whole animal near-infrared imaging.
Imaging was conducted on a whole animal imaging platform (Li-Cor, Lincoln NE) under continuous isoflurane anesthesia and normothermic control. Prior to starting the imaging protocol, 10 nm of ZW800-1 was dissolved in 0.9% sodium chloride solution, and 5 mg of FITC-inulin were simultaneously injected via the intrajugular catheter in 500 µl total volume. During protocol development, four consecutive post-CA/CPR mice injected with 10 mg FITC-inulin died immediately. Thereafter, we halved the dose of FITC-inulin. Near-infrared images (excitation 785 nm, emission 820 nm) were acquired at time 0 and every 5 min for 120 min. 55, 75, 95, and 120 min after injection, the animal carrier was externalized from the imaging platform, and <40 µl blood sample was acquired into a heparinized microcapillary tube from the tail vein. Emission at 530 nm, measured on a plate reader, was quantified using a standard curve. To generate ZW800-1t1/2, one-phase decay curves were fit to normalize fluorescence values from 55 to 120 min after bolus injection. Since ZW800-1 near-infrared fluorescence is not attenuated by skin and subcutaneous tissue, a plasma standard curve was used to calculate ZW800-1 concentration from fluorescence. This generated values that agreed with values calculated using the precalculated fluorescence-concentration relationship method of Shock-Kusch (34).
Bilateral renal pedicle occlusion and immediate measurement of ZW800-1 fluorescence.
Under isoflurane general anesthesia, right and left renal pedicles were suture-ligated via flank incisions. Immediately following ligation of the second renal pedicle, 10 nmol of ZW800-1 in 0.9% sodium chloride solution was injected via the tail vein, and sequential whole animal imaging was performed as described previously.
Measurement of ZW800-1 bolus dispersion rate.
To test the hypothesis that fluorescence dispersion after ZW800 bolus injection was related to cardiac function, we used 1 Hz near-infrared imaging starting immediately before rapid (5 s) injection of 10 nM ZW800-1. Imaging continued 10 min after bolus injection. We analyzed the time course of fluorescence increase at seven regions of interest (ROIs) and fit curves to the fluorescence data. Injection duration and start time were fluorimetrically determined using an ROI over the catheter tip at the entry point.
Measurement of serum decay of ZW800-1 fluorescence.
ZW800-1 samples were diluted in PBS (control) or mouse serum (Innovative Research, Novi, MI) and incubated at 37°C for 4 h (n = 16). Fluorescence was measured hourly on a plate reader and expressed as a percentage of baseline fluorescence. To confirm the in vitro findings in vivo and to develop relevant correction, mice (n = 3) were exsanguinated under isoflurane anesthesia, serum was separated using centrifugation, and pooled. ZW800-1 and serum (or PBS control) were mixed in concentrations between 0 and 2.5 µM on 96-well plates and imaged immediately, and hourly for 4 h. Fluorescence decay curves were generated and modeled for each concentration in Prism 6.0 with linear and one-phase decay models. Goodness-of-fit parameters were compared to select the model that best fit the data.
Measurement of tubular epithelial cell death.
To determine the extent of renal injury, we determined the volume of tubular necrosis (volume of necrotic tubules, VNT) using unbiased stereology to quantify dead tubular epithelial cells on serial renal sections relative to the reference volume of the kidney, as described previously (21, 26). In addition, in a separate cohort (n = 10), we stained widely separated renal sections for activated caspase 3, 6 h after CA/CPR, quantifying caspase-positive cells per mm2 total section area.
Statistical analysis.
Two group differences were assessed using (two-tailed) t-tests with significance inferred from P < 0.05. Correlation analysis was conducted using Spearman’s coefficient for normally distributed results and Pearson’s for non-normal distributions. A two-way ANOVA with two-group repeated-measures analysis was performed with the Sidak test for multiple comparisons. To test agreement between GFR and ZW800-1 clearance measured transcutaneously, we performed Bland-Altman analysis (3), and to quantify departure from agreement, we used polar analysis (12, 13). Results are presented in the text and figures as means ± SD or with 95% confidence intervals, as appropriate.
RESULTS
CA/CPR leads to experimental cardiorenal syndrome type 1.
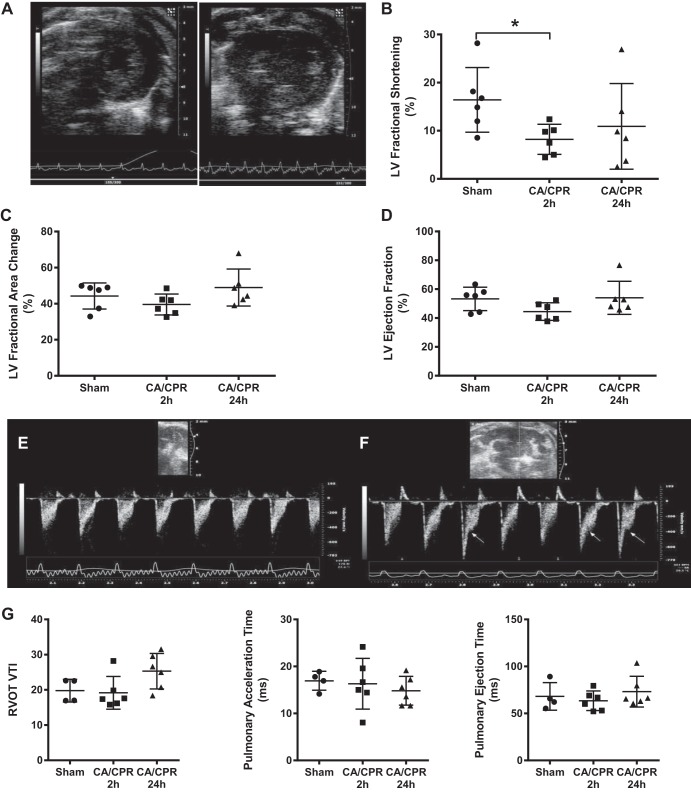
Potassium chloride induced immediate cardiac arrest in all mice. Survival to protocol completion was 100% after sham, 47% after 10 min CA, and 83% after 8 min CA. CA/CPR caused a transient, profound, interruption of cardiac function. Two hours after return of spontaneous circulation (ROSC), myocardial function was already recovering, and LV and RV function recovered and was indistinguishable from sham by 24 h after ROSC. LV fractional shortening was halved in CA/CPR mice 2 h after ROSC (8.2 ± 3.1 compared with 16.4 ± 6.7% in shams; P = 0.03, n = 12, illustrated in Supplemental Videos S1 and S2), but LV ejection fraction and fractional area change were not significantly different from sham (all supplemental material for this article is accessible on the journal web site). Right ventricular function was also altered 2 h after CA/CPR, as demonstrated by transient systolic flow reductions (“notching”) observed in all CA/CPR animals but in none of the sham animals. Pulmonary ejection time, pulmonary acceleration time, and RVOT VTI were not significantly different 2 h after CA/CPR. These results are summarized in Fig. 1.
Fig. 1.
Cardiac function 2 h and 24 h after cardiac arrest and cardiopulmonary resuscitation (CA/CPR). A: representative end-diastolic transthoracic short-axis view from sham (left) and post cardiac arrest (right) mouse, 2 h after resuscitation. B: left ventricular (LV) fractional shortening was significantly decreased 2 h after, but not 24 h after CA/CPR. C and D: fractional area change and LV ejection fraction were not significantly different from sham 2 h after or 24 h after CA/CPR. E and F: Doppler trace from the proximal pulmonary artery (PA) 2 h after sham (E) and CA/CPR (F). Arrows point to midsystolic flow reduction (“notching”) in the trace, indicating abnormal right ventricular afterload. G: right ventricular outflow tract (RVOT) velocity-time integral (VTI), pulmonary acceleration time, and pulmonary ejection time were not significantly different between groups.
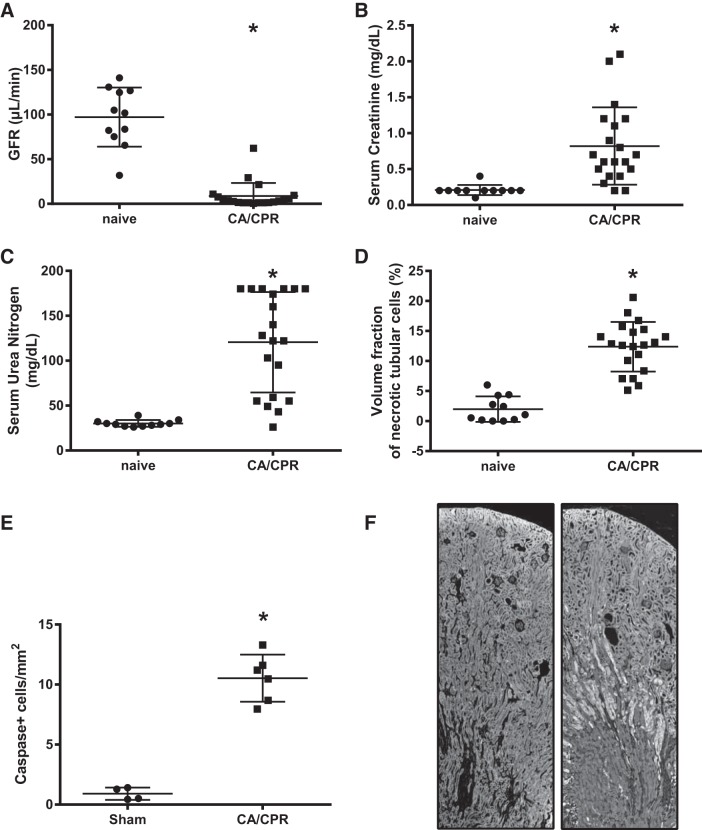
Acute cardiac dysfunction in the murine CA/CPR model leads to renal failure, including near-zero GFR 24 h after CA/CPR (8.8 ± 14.6 compared with sham 97.1 ± 33.0 μl/min; P < 0.0001, n = 31). The reduction in GFR is associated with an order of magnitude increment in tubular caspase staining (10.5 ± 2.0 vs. 0.9 ± 0.5 cells/mm2; P < 0.0001, n = 10) and increased tubular cell death measured by unbiased stereology (12.4 ± 1.3 vs. 1.9 ± 2.1%; P < 0.0001, n = 31), as well as increased serum urea nitrogen (120.6 ± 55.9 vs. 30.9 ± 3.7 mg/dl) and creatinine (0.8 ± 0.5 vs 0.2 ± 0.1 mg/dl; P = 0.0009, n = 31). Acute (24 h after CA/CPR) tubular cell death, as demonstrated by Fluoro-jade B-positive staining, is most apparent at the corticomedullary junction, similar to findings in renal biopsy studies of patients with AKI (15, 38). These results are summarized in Fig. 2.
Fig. 2.
Acute kidney injury 24 h after cardiac arrest and cardiopulmonary resuscitation (CA/CPR). A: glomerular filtration rate (GFR), measured by serial assay after bolus injection of FITC-inulin, is near-zero. B: serum creatinine is elevated nearly ×10 baseline values. C: serum urea nitrogen is similarly increased. D: CA/CPR causes significant tubular cell death as quantified by unbiased stereology. E: ×10 increase in tubular epithelial cell death 24 h after CA/CPR indicates significant tubular apoptosis. F: representative mouse kidney sections, stained with fluoro-jade B, fixed 24 h after sham (left) or CA/CPR (right) procedure, demonstrating selective injury of corticomedullary tubules.
ZW800-1 biodistribution is exclusively renal.
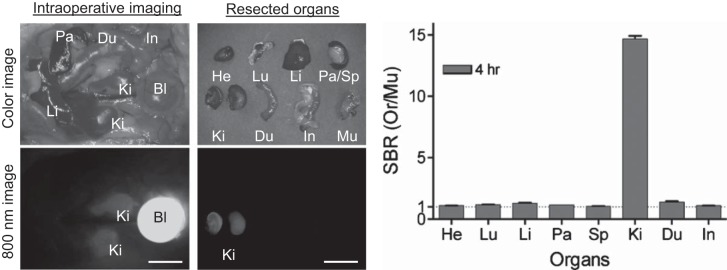
Intraoperative 800-nm imaging of CD-1 mice 4 h after ZW800-1 bolus injection demonstrated bright fluorescence in the bladder and kidneys (Fig. 3). Shadowing of the cranial renal poles by omental fat and the liver indicate the renal specificity of the fluorophore. The fluorescence signal/background in kidney was 14× greater than that of heart, lung, liver, pancreas, spleen, duodenum, or large intestine, indicating that following bolus administration and clearance from the plasma, ZW800-1 is entirely confined to the urinary system.
Fig. 3.
In vivo biodistribution and clearance of ZW800-1 in mice: 10 nmol (0.2 mg/kg) of ZW800-1 was injected intravenously into 20 g CD-1 mice 4 h before imaging (n = 3). Major organs were resected after intraoperative imaging at 4 h postinjection. The signal-to-background ratio (SBR) was calculated as SBR = fluorescence/background, where background is the fluorescence intensity of muscle. Bl, bladder; Du, duodenum; He, Heart; In, intestine; Ki, kidneys; Li, liver; Lu, lungs; Mu, muscle; Pa, pancreas; Sp, spleen. Scale bars = 1 cm. Exposure time = 100 ms.
ZW800-1 is not cleared in mice subjected to bilateral nephrectomy.
Glomerular filtration rate was 0 ± 0 µl/min in nephrectomized female mice (n = 4). Mean fluorescence elimination half time for FITC-inulin was 510 ± 277 min, while that of ZW800-1 in the same animals was 246 ± 63 min with nonexponential, nonlinear terminal kinetics, suggesting very prolonged clearance. During 2-h imaging intervals, no visible change in bladder fluorescence was observed, and no urine was produced. As previous data indicate no significant hepatic, pulmonary, or gastrointestinal clearance of ZW800-1, we investigated whether ZW800-1 fluorescence decay in serum accounted for the observed difference between GFR and ZW800-1 fluorescence elimination half time in nephrectomized mice.
ZW800-1 fluorescence decay in mouse serum.
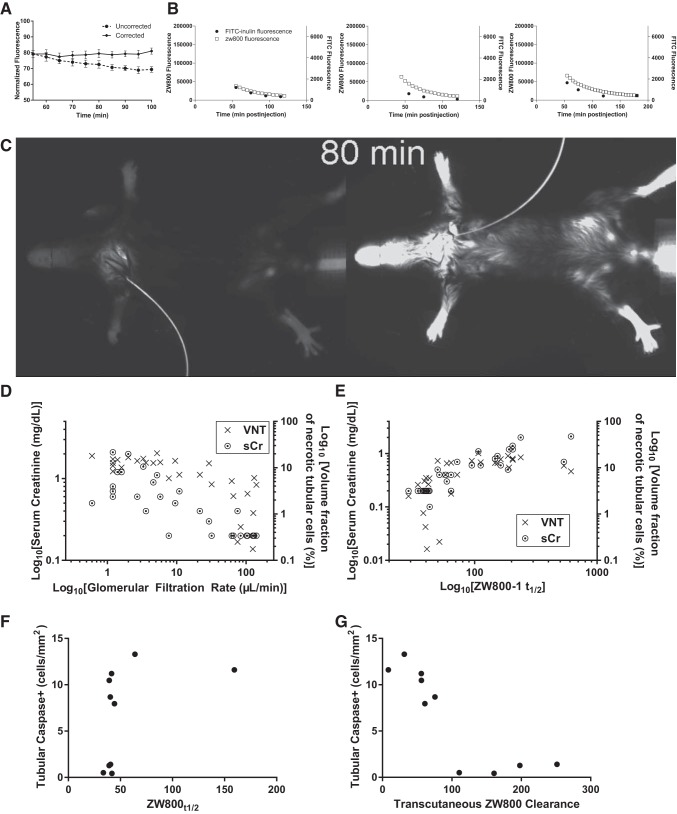
Serum samples from mice injected with ZW800-1, which were extensively handled at room temperature demonstrated significantly reduced fluorescence compared with transcutaneous fluorescence from identically injected mice. As the most likely explanation was decay of fluorescence in the isolated serum, we measured this in vitro. The rate of fluorescence decay in serum exceeded that in PBS by 35% (n = 16, P = 0.03). When repeated using the in vivo imaging equipment and pooled serum from sex-, strain-, circadian- and chow-identical mice to the other mice in this investigation, the decay rate was similar. Accordingly, we applied a correction based on this in vivo rate of decay to the measured fluorescence decay in nephrectomized mice, demonstrating complete absence of renal clearance (Fig. 4A).
Fig. 4.
ZW800-1 fluorescence after bolus injection in nephrectomized (A), and cardiac arrest/cardiopulmonary resuscitation (CA/CPR)- or sham-treated mice (B–G). A: ZW800-1 fluorescence after bolus injection in four surgically naïve mice. The reduction in uncorrected fluorescence over time (~10% of baseline fluorescence) is eliminated by application of correction for serum-mediated decay of ZW800-1 fluorescence. Error bars are standard deviation. B: transcutaneous ZW800-1 fluorescence (□) and FITC-inulin serum fluorescence in three individual animals demonstrating close apposition of time-signal curves. C: 800 nm in vivo fluorescence image of sham (left) and CA/CPR-treated mouse. This still image from Supplemental Video S3 was taken 80 min after bolus injection of ZW800-1. Intact renal clearance of ZW800-1 is demonstrated in the sham by lack of transcutaneous fluorescence, in contrast to the CA/CPR-treated mouse (right), which exhibits bright fluorescence due to lack of clearance of the bolus. D: glomerular filtration rate (GFR) 24 h after sham or CA/CPR closely correlates with serum creatinine (left axis) and unbiased stereology-quantified tubular cell death. E: relationship in D is recapitulated between ZW800-1 fluorescence half-time ZW800-1t1/2 and serum creatinine (r = 0.89, P < 0.0001) and tubular cell death (r = 0.74, P < 0.0001). In D and E, data are log-transformed to demonstrate correlation as shown; analysis in text is of untransformed primary data. F and G: Caspase-positive cells/mm2 in histologic section from 10 mice subjected to sham or CA/CPR, 10 h after respective procedures. The half time for ZW800-1 fluorescence (ZW800-1t1/2) did not correlate well with tubular caspase signal (F); however, ZW800-1 clearance (G) correlates strongly with tubular caspase, r = −0.87, P = 0.0022.
ZW800-1 fluorescence half-time and clearance correlate with serum creatinine and tubular epithelial cell death after CA/CPR.
To determine whether transcutaneous measurements of ZW800-1 demonstrated utility as a noninvasive test for acute kidney injury in a disease model, we compared ZW800-1 fluorescence disappearance half-time (ZW800-1t1/2) with serum creatinine, serum urea nitrogen, and tubular cell death measured by unbiased stereologic assessment of flourojade-B-stained histologic sections, as well as caspase-3 staining for tubular apoptosis (Fig. 4, B–G). There was strong correlation between ZW800-1t1/2 and both histologic and functional measures of tubular cell death (r = 0.74, 95% CI 0.51–0.87 for VNT, P < 0.0001 and 0.89 95% CI 0.89–0.95 for both sCr and sUN, respectively, n = 31). Tubular caspase-3 10 h after CA/CPR did not correlate significantly with ZW800-1t1/2 (r = 0.55; P = 0.11, n = 10); however, transcutaneously measured ZW800-1 clearance correlated well with tubular caspase (r = −0.87; P = 0.0022, n = 10). These data indicate that after CA/CPR, ZW800-1t1/2 correlates well with renal functional and histologic outcome overall, but not specifically with apoptosis as measured at 10 h postreperfusion, while transcutaneous fluorescence-derived ZW800-1 clearance correlates with serum measures of renal injury and both necrotic and apoptotic tubular cell death.
ZW800-1 clearance measured by transcutaneous fluorescence is a noninvasive measure of glomerular filtration rate.
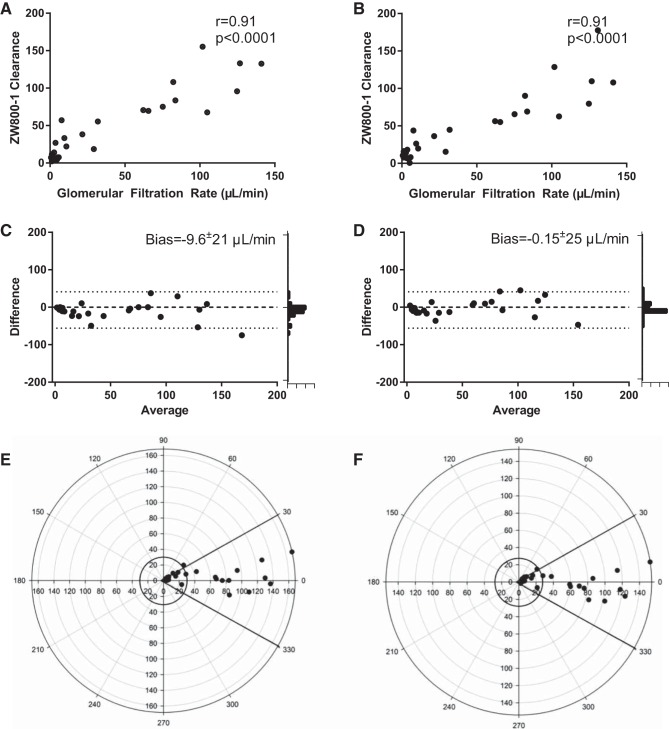
In early experiments, ZW800-1 fluorescence decay curves closely matched simultaneous serum sample-derived FITC-inulin decay curves. Across a wide spectrum of renal injury and in male, female, young, and aged mice, the transcutaneous fluorescence clearance of ZW800-1 very closely matched the GFR (illustrated in Supplemental Video S3 and summarized in Fig. 5). The correlation between GFR and ZW800-1 clearance was high overall (r = 0.91, 95% CI 0.83–0.96, P < 0.0001, n = 31), and subgroup correlations were all >0.7 (males: r = 0.74, n = 16, P < 0.001; females: r = 0.86, n = 15, P < 0.001; <20 wk: r = 0.88, n = 16, P < 0.001; >20 wk: r = 0.84, n = 15, r = 0.85). Because correlation analysis indicates level of association, but not agreement, between two hypothetically equivalent measures, we also performed Bland-Altman analysis on measured values and on measured values to which correction for serum decay had been applied. This revealed close agreement between GFR and transcutaneously measured clearance of ZW800-1 with a bias of −9.6 ± 21 µl/min and 95% limits of agreement −52 to 32 µl/min. Correction for serum decay reduced bias to −2.8 ± 20 μl/min and 95% limits of agreement −42–36 μl/min. We used polar plots, which quantify divergence from the gold standard, to test agreement between transcutaneous ZW800-1 clearance and GFR. The uncorrected ZW800-1 clearance demonstrated a mean polar angle of 6.08° with radial limits (95% CI) between −0.30 and 12.45°. The corrected ZW800-1 vs. GFR had a mean polar angle of −2.36° with radial limits (95% CI) between −6.73 and 2.01°, indicating close agreement at baseline, improved by correction for serum decay. Therefore, ZW800-1 clearance, measured transcutaneously, agrees with a gold-standard measure of GFR.
Fig. 5.
Results from 31 paired measurements in sham- and cardiac/arrest and cardiopulmonary resuscitation-treated mice demonstrate that transcutaneously measured ZW800-1 clearance is a measurement of glomerular filtration rate (GFR). A: uncorrected ZW800-1 clearance correlates with GFR, measured using serial measurement of serum FITC-inulin concentration after bolus injection. B: correlation between GFR and ZW800-1 is not quantitatively improved when ZW800-1 fluorescence is corrected for serum decay. C: Bland-Altman analysis of uncorrected ZW800-1 clearance compared with GFR demonstrates a biologically insignificant bias of −9.6 ± 21 µl/min with 95% limits of agreement: −52 to 32 µl/min. Correction for serum decay (D) reduced bias to −0.15 ± 25 μl/min with 95% limits of agreement: −42 - 36 μl/min. Histograms on the right demonstrate results converge on agreement. Polar plot of uncorrected (E) and corrected (F) ZW800-1 clearance compared with GFR. The uncorrected mean polar angle is 6.08 degrees with radial limits (95% CI) −0.30–12.45°. The corrected mean polar angle was −2.36° with radial limits (95% CI) between −6.73 and 2.01°, indicating agreement.
ZW800-1 transcutaneous fluorescence distribution time is reduced by left ventricular impairment after murine CA/CPR.
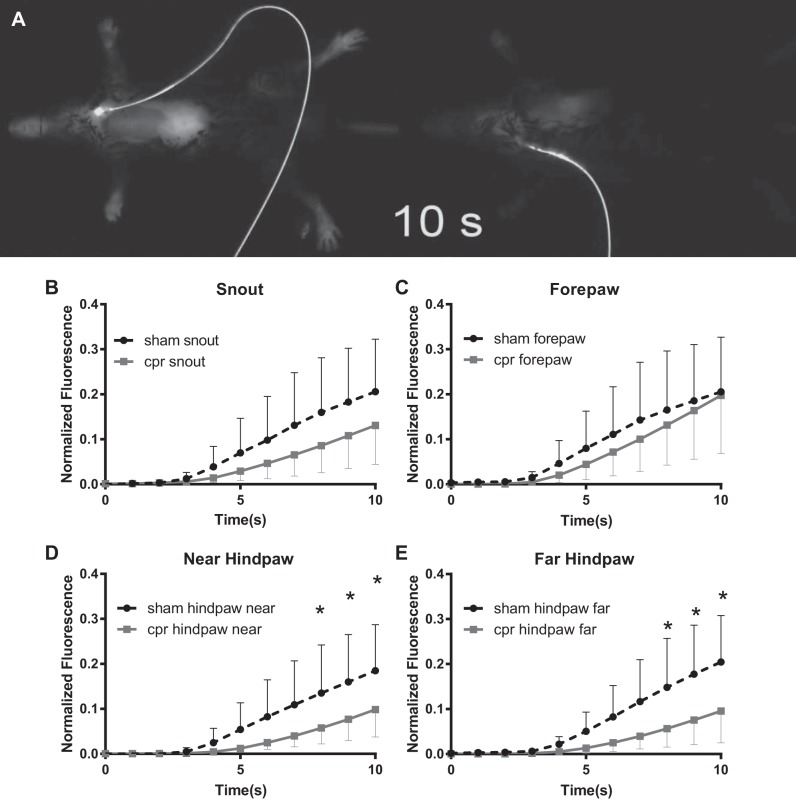
During the renal function experiments, we noted that the time for the spread of ZW800-1 fluorescence signal from injection site to anatomically distant sites on the mouse was delayed in CA/CPR mice compared with sham. The increase in fluorescence between 1 and 10 min after injection in the renal function protocol (with images taken every 5 min) was <30% that of sham in CA/CPR mice (slope of 1–10 min line 576 ± 259 vs. 1768 ± 1936 AFU/min, n = 31, P = 0.01). Therefore, we performed 1 Hz imaging after bolus injection. There was no difference between groups in bolus duration. Fluorescence values were significantly different between sham and CA/CPR starting 8 s after injection at both hindpaws with the maximal difference at the far (left) hindpaw (10-s mean difference 0.11 AFU, 95% CI 0.03–0.19; P = 0.003 n = 12) Mathematical modeling of the time-fluorescence curve revealed best fit with a second-order polynomial model (sham group: R2, 0.5742; CA/CPR group: R2, 0.552) compared with exponential growth (sham group: R2, 0.550; CA/CPR group; R2, 0.539) and a first-order polynomial model (sham group: R2, 0.534; CA/CPR group: R2, 0.473). Individual second-order polynomial curve modeling revealed significant correlation between the B1 coefficient and TTE-derived fractional shortening (r = 0.74, CI 0.29–0.92; P = 0.006, n = 12) and halving in magnitude due to CA/CPR (B1 0.009 ± 0.007 vs. sham B1 0.02 ± 0.01; P = 0.03, n = 12). Together, these data demonstrate feasibility of modeling cardiac function from ZW800-1 bolus dispersion curves, and suggest it is possible to measure both cardiac and renal function from the same bolus injection. These results are summarized in Fig. 6 and are illustrated in Supplemental Video S4.
Fig. 6.
Transcutaneous fluorescence bolus distribution time measurements. A: still from 1 Hz recording of 800-nm fluorescence starting at bolus injection (see Supplemental Video S4). At 10 s after injection, sham (left) demonstrates widespread fluorescence, indicating extensive distribution of ZW800-1. Cardiac arrest and cardiopulmonary resuscitation-treated mouse (right) demonstrates reduced rate of distribution, the snout and chest have just begun to exhibit fluorescence. B–E: plots of time-density relationship for ZW800-1 fluorescence at 1 Hz imaging frequency for 10 s after bolus injection. The distal hind extremity regions of interest demonstrated significant differences in fluorescence starting 8 s after injection.
DISCUSSION
The central finding of this study is that the novel near-infrared fluorophore ZW800-1 is a robust indicator of renal function in male, female, young, old, healthy, and critically ill mice. A strength of these experiments is extensive investigation across a spectrum of renal injury using rigorous methods, including unbiased stereology (26), thus, demonstrating an association between tubular epithelial cell death and the novel metric of renal function. Development of rapid, noninvasive, and nontoxic methods to measure GFR is critical to translational research in kidney disease. ZW800-1 is a nontoxic synthetic molecule with MW<1,000, which exhibits high quantum yield, bright near-infrared fluorescence, and near-zero background fluorescence unlike all other proposed fluorescent markers of GFR. ZW800-1 fluorescence is not significantly attenuated by tissue or hemoglobin, addressing a limitation of use of visible-range indicators, such as FITC for transcutaneous measurement. In addition, ZW800-1 is currently in Phase I human trials for use in cancer imaging (9), rendering it particularly useful in translational studies. Our work builds on other developmental noninvasive measures of GFR, including scintigraphy using 99mTc-DTPA (30) and fluorimetry, using visible-range indicators, such as FITC-inulin and FITC-sinistrin (29, 33, 34). Glomerular filtration measured by FITC-inulin assay in our sham mice is in line with published values from male mice under anesthesia (28), although we measured across a wide spectrum of age. ZW800-1 fluorescence half time and clearance both correlated with creatinine, tubular injury, and GFR. In this study, ZW800-1 clearance correlated closely with GFR, demonstrating close agreement by both Bland-Altman and polar analysis. Because serum ZW800-1 serum decay affected the observed clearance in nephrectomized mice, we applied a correction for serum decay to ZW800-1 clearance measurements. Although this correction reduced bias, it primarily affected measurements in mice with very severe renal impairment, in which small differences of GFR have little biologic meaning; therefore, correction for serum decay is not necessary when measuring GFR with ZW800-1 transcutaneous clearance.
To test the novel indicator in a translationally relevant model, we employed murine normothermic CA/CPR, which recapitulates the clinical correlate, whole-body ischemia-reperfusion followed by AKI. Definitions in cardiorenal syndrome are challenged by incomplete understanding of pathophysiology. A working definition based on acuity and kidney or heart primacy in dysfunction is currently most widely used, but is controversial because of lack of mechanistic underpinnings (6). CRS1 is defined as acute kidney dysfunction caused by acute cardiac dysfunction, which is precisely a characteristic of cardiac arrest followed by cardiopulmonary resuscitation (16, 36). We propose that CA/CPR is a model of CRS1, in which necessary detailed mechanistic studies may be carried out. The data we present demonstrate the acute, transient nature of cardiac dysfunction caused by CA/CPR and the profound effect on renal function and histopathology in a model that allows genetic, physiological, and pharmacologic manipulation (21, 22). Surprisingly, animal models to study cardiorenal syndrome are limited. The most robust work has been performed in chronic kidney disease models (5) and chronic heart failure models (32). Our prior work and that of another group demonstrate increased creatinine and renal inflammation after CA/CPR (7), but measurement of GFR, association with renal pathology, echocardiography, and near-infrared fluorimetry have not been performed to date. This study provides essential data to support the use of CA/CPR in studies investigating CRS1, which increasingly limits survival in patients whose lives are saved by advanced treatment for acute cardiovascular illness (8, 23).
Our serendipitous finding that the early distribution of fluorescence after bolus injection of ZW800-1 correlates with LV function was facilitated by the use of new, whole animal rapid near-infrared imaging technology and by simultaneous evaluation of a cardiorenal model in which we were already performing echocardiography. We hypothesized that cardiac function was driving the observed difference in time-to-appearance of fluorescence in sham and CA/CPR-treated mouse hindpaws. Indicator dilution is a commonly used method to measure cardiac output, which is proportional to the area under the curve of a rapidly diluted indicator; for example, the near-infrared fluorophore indocyanine green (24). However, measurement of renal clearance requires dosing that renders indicator dilution unmeasurable. As combined measurement of cardiac and renal function is desirable, we used time-density curve modeling to evaluate the maximum density gradient (i.e., the relative rate-of-rise) parameter after bolus injection in sham and CA/CPR-subjected mice. The distribution and subsequent acquisition of signal from intravenously injected indicator are primarily dependent on flow (4). Since near-infrared fluorescence is minimally attenuated by skin and subcutaneous tissue, transcutaneous fluorescence dispersion of a near-infrared flourophore bolus is determined by flow. In animals with cardiac functional impairment, pulmonary edema may result, and it is conceivable that this could interfere with interpretations of arterial flow in experiments such as ours, in which the bolus is intravenously administered. However, if the primary restriction to flow in CA/CPR animals were due to pulmonary edema, one would expect the arterial distribution to be equivalently delayed to forelimb, snout, and hindlimb in CA/CPR animals relative to sham. Our data demonstrate, however, that CA/CPR induces delayed dispersion of the ZW800-1 bolus to the distal extremity but not the snout and forelimb and that the distal dispersion rate correlates with cardiac function. As the mouse vasculature is visible in the images from this investigation (Fig. 6), with improved imaging protocols, blood velocity and flow may be derived from time-density curves (1), yielding precise estimates of cardiac function from the same injection used to measure renal function. We speculate that the time-density relationship may be used to indicate vascular flow. Combined with the demonstrated relationship between distal dispersion and left ventricular function, it is likely that the necessary signal is present for ZW800-1 transcutaneous fluorescence to be used to indicate left ventricular function. An important limitation of this interpretation is that due to experimental constraints in critically ill mice, we performed limited transthoracic echocardiography. This early data will serve to inform further investigation focused on combined functional assessment.
Our study has additional limitations. We studied experimental cardiorenal syndrome in a mouse model, and whether the clinical condition and the mouse model coincide, and to what extent, is unknown. Although we found that GFR and other measures of renal function in our model correspond with clinical findings, there is a surprising lack of renal biopsy studies in humans with CRS1, which, as has been recently noted (14), would greatly aid mechanistic studies in animal models. An additional limitation of our study is that serum clearance methods require repeated measurement over time and, therefore, characterize what may be dynamic change with static quantities—measurements are made after the fact. Measurements of transcutaneous ZW800 clearance could fail to correspond to serum clearance if there is differential tissue attenuation of fluorescence during the period of image acquisition. As it is highly unlikely that the very bright fluorescence of ZW800-1 would be altered by physiological changes in tissue attenuation during the maximally 2-h period of measurement, we did not assess this possibility quantitatively.
We conclude that CA/CPR leads to experimental CRS, a model that may enable detailed study of this condition. In this model, transcutaneous clearance of ZW800-1 is a measurement of GFR, and transcutaneous ZW800-1 time-density curve modeling may be a feasible method to estimate cardiac function.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.I., R.W., K.J.S., J.H.L., and M.P.H. performed experiments; M.I., R.W., K.J.S., T.S., J.H.L., M.B.M., and M.P.H. analyzed data; M.I., J.H.L., M.B.M., H.S.C., S.A., and M.P.H. interpreted results of experiments; M.I., R.W., T.S., M.B.M., H.S.C., and M.P.H. prepared figures; M.I. and M.P.H. drafted manuscript; M.I., M.B.M., H.S.C., S.A., and M.P.H. edited and revised manuscript; M.I., R.W., K.J.S., T.S., J.H.L., M.B.M., H.S.C., S.A., and M.P.H. approved final version of manuscript.
GRANTS
This article was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant DK-090754 to M. P. Hutchens.
REFERENCES
- 1.Barfett JJ, Velauthapillai N, Fierstra J, Crawley A, Coolens C, Crean A, Jaskolka J, Dufort P, Krings T, Mikulis D. Intra-vascular blood velocity and volumetric flow rate calculated from dynamic 4D CT angiography using a time of flight technique. Int J Cardiovasc Imaging 30: 1383–1392, 2014. doi: 10.1007/s10554-014-0471-3. [DOI] [PubMed] [Google Scholar]
- 2.Bauman LA, Watson NE Jr, Scuderi PE, Peters MA. Transcutaneous renal function monitor: precision during unsteady hemodynamics. J Clin Monit Comput 14: 275–282, 1998. doi: 10.1023/A:1009992308204. [DOI] [PubMed] [Google Scholar]
- 3.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 327: 307–310, 1986. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 4.Blomley MJ, Dawson P. Bolus dynamics: theoretical and experimental aspects. Br J Radiol 70: 351–359, 1997. doi: 10.1259/bjr.70.832.9166070. [DOI] [PubMed] [Google Scholar]
- 5.Bongartz LG, Braam B, Gaillard CA, Cramer MJ, Goldschmeding R, Verhaar MC, Doevendans PA, Joles JA. Target organ cross talk in cardiorenal syndrome: animal models. Am J Physiol Renal Physiol 303: F1253–F1263, 2012. doi: 10.1152/ajprenal.00392.2012. [DOI] [PubMed] [Google Scholar]
- 6.Braam B, Joles JA, Danishwar AH, Gaillard CA. Cardiorenal syndrome—current understanding and future perspectives. Nat Rev Nephrol 10: 48–55, 2014. doi: 10.1038/nrneph.2013.250. [DOI] [PubMed] [Google Scholar]
- 7.Burne-Taney MJ, Kofler J, Yokota N, Weisfeldt M, Traystman RJ, Rabb H. Acute renal failure after whole body ischemia is characterized by inflammation and T cell-mediated injury. Am J Physiol Renal Physiol 285: F87–F94, 2003. doi: 10.1152/ajprenal.00026.2003. [DOI] [PubMed] [Google Scholar]
- 8.Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, Kimmel PL. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol 9: 448–456, 2014. doi: 10.2215/CJN.02440213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi HS, Gibbs SL, Lee JH, Kim SH, Ashitate Y, Liu F, Hyun H, Park G, Xie Y, Bae S, Henary M, Frangioni JV. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat Biotechnol 31: 148–153, 2013. doi: 10.1038/nbt.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi HS, Nasr K, Alyabyev S, Feith D, Lee JH, Kim SH, Ashitate Y, Hyun H, Patonay G, Strekowski L, Henary M, Frangioni JV. Synthesis and in vivo fate of zwitterionic near-infrared fluorophores [International ed. English]. Angew Chem Int Ed Engl 50: 6258–6263, 2011. doi: 10.1002/anie.201102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colton IJ, Carbeck JD, Rao J, Whitesides GM. Affinity capillary electrophoresis: a physical-organic tool for studying interactions in biomolecular recognition. Electrophoresis 19: 367–382, 1998. doi: 10.1002/elps.1150190303. [DOI] [PubMed] [Google Scholar]
- 12.Critchley LA, Lee A, Ho AM. A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg 111: 1180–1192, 2010. doi: 10.1213/ANE.0b013e3181f08a5b. [DOI] [PubMed] [Google Scholar]
- 13.Critchley LA, Yang XX, Lee A. Assessment of trending ability of cardiac output monitors by polar plot methodology. J Cardiothorac Vasc Anesth 25: 536–546, 2011. doi: 10.1053/j.jvca.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 14.de Caestecker M, Humphreys BD, Liu KD, Fissell WH, Cerda J, Nolin TD, Askenazi D, Mour G, Harrell FE Jr, Pullen N, Okusa MD, Faubel S, Group AAA; ASN AKI Advisory Group . Bridging translation by improving preclinical study design in AKI. J Am Soc Nephrol 26: 2905–2916, 2015. doi: 10.1681/ASN.2015070832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas M, Spargo BH, Wit EJ, Meehan SM. Etiologies and outcome of acute renal insufficiency in older adults: a renal biopsy study of 259 cases. Am J Kidney Dis 35: 433–447, 2000. doi: 10.1016/S0272-6386(00)70196-X. [DOI] [PubMed] [Google Scholar]
- 16.Hasper D, von Haehling S, Storm C, Jörres A, Schefold JC. Changes in serum creatinine in the first 24 hours after cardiac arrest indicate prognosis: an observational cohort study. Crit Care 13: R168, 2009. doi: 10.1186/cc8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutchens MP, Fujiyoshi T, Koerner IP, Herson PS. Extracranial hypothermia during cardiac arrest and cardiopulmonary resuscitation is neuroprotective in vivo. Ther Hypothermia Temp Manag 4: 79–87, 2014. doi: 10.1089/ther.2014.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutchens MP, Fujiyoshi T, Komers R, Herson PS, Anderson S. Estrogen protects renal endothelial barrier function from ischemia-reperfusion in vitro and in vivo. Am J Physiol Renal Physiol 303: F377–F385, 2012. doi: 10.1152/ajprenal.00354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutchens MP, Kosaka Y, Zhang W, Fujiyoshi T, Murphy S, Alkayed N, Anderson S. Estrogen-mediated renoprotection following cardiac arrest and cardiopulmonary resuscitation is robust to GPR30 gene deletion. PLoS One 9: e99910, 2014. doi: 10.1371/journal.pone.0099910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchens MP, Nakano T, Kosaka Y, Dunlap J, Zhang W, Herson PS, Murphy SJ, Anderson S, Hurn PD. Estrogen is renoprotective via a nonreceptor-dependent mechanism after cardiac arrest in vivo. Anesthesiology 112: 395–405, 2010. doi: 10.1097/ALN.0b013e3181c98da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchens MP, Nakano T, Kosaka Y, Dunlap J, Zhang W, Herson PS, Murphy SJ, Anderson S, Hurn PD. Estrogen is renoprotective via a nonreceptor-dependent mechanism after cardiac arrest in vivo. Anesthesiology 112: 395–405, 2010. doi: 10.1097/ALN.0b013e3181c98da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikeda M, Swide T, Vayl A, Lahm T, Anderson S, Hutchens MP. Estrogen administered after cardiac arrest and cardiopulmonary resuscitation ameliorates acute kidney injury in a sex- and age-specific manner. Crit Care 19: 332, 2015. doi: 10.1186/s13054-015-1049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Cai L, Liang X, Du Z, Chen Y, An S, Tan N, Xu L, Li R, Li L, Shi W. Identification and predicting short-term prognosis of early cardiorenal syndrome type 1: KDIGO is superior to RIFLE or AKIN. PLoS One 9: e114369, 2014. doi: 10.1371/journal.pone.0114369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maarek JM, Holschneider DP, Rubinstein EH. Fluorescence dilution technique for measurement of cardiac output and circulating blood volume in healthy human subjects. Anesthesiology 106: 491–498, 2007. doi: 10.1097/00000542-200703000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Molitoris BA, Reilly ES. Quantifying glomerular filtration rates in acute kidney injury: a requirement for translational success. Semin Nephrol 36: 31–41, 2016. doi: 10.1016/j.semnephrol.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyengaard JR. Stereologic methods and their application in kidney research. J Am Soc Nephrol 10: 1100–1123, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Opotowsky AR, Clair M, Afilalo J, Landzberg MJ, Waxman AB, Moko L, Maron BA, Vaidya A, Forfia PR. A simple echocardiographic method to estimate pulmonary vascular resistance. Am J Cardiol 112: 873–882, 2013. doi: 10.1016/j.amjcard.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 286: F590–F596, 2004. doi: 10.1152/ajprenal.00324.2003. [DOI] [PubMed] [Google Scholar]
- 29.Rabito CA, Chen Y, Schomacker KT, Modell MD. Optical, real-time monitoring of the glomerular filtration rate. Appl Opt 44: 5956–5965, 2005. doi: 10.1364/AO.44.005956. [DOI] [PubMed] [Google Scholar]
- 30.Rabito CA, Moore RH, Bougas C, Dragotakes SC. Noninvasive, real-time monitoring of renal function: the ambulatory renal monitor. J Nucl Med 34: 199–207, 1993. [PubMed] [Google Scholar]
- 31.Rabito CA, van Tongeren S, Zavorskas PA, Stricker-Krongrad A, Robb J, Haupert GT Jr. Measurement of glomerular filtration rate in anesthetized and conscious rhesus monkeys (Macaca mulatta). Am J Vet Res 71: 1492–1499, 2010. doi: 10.2460/ajvr.71.12.1492. [DOI] [PubMed] [Google Scholar]
- 32.Rafiq K, Noma T, Fujisawa Y, Ishihara Y, Arai Y, Nabi AHMN, Suzuki F, Nagai Y, Nakano D, Hitomi H, Kitada K, Urushihara M, Kobori H, Kohno M, Nishiyama A. Renal sympathetic denervation suppresses de novo podocyte injury and albuminuria in rats with aortic regurgitation. Circulation 125: 1402–1413, 2012. doi: 10.1161/CIRCULATIONAHA.111.064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schock-Kusch D, Geraci S, Ermeling E, Shulhevich Y, Sticht C, Hesser J, Stsepankou D, Neudecker S, Pill J, Schmitt R, Melk A. Reliability of transcutaneous measurement of renal function in various strains of conscious mice. PLoS One 8: e71519, 2013. doi: 10.1371/journal.pone.0071519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schock-Kusch D, Sadick M, Henninger N, Kraenzlin B, Claus G, Kloetzer HM, Weiss C, Pill J, Gretz N. Transcutaneous measurement of glomerular filtration rate using FITC-sinistrin in rats. Nephrol Dial Transplant 24: 2997–3001, 2009. doi: 10.1093/ndt/gfp225. [DOI] [PubMed] [Google Scholar]
- 35.Steinbach S, Krolop N, Strommer S, Herrera-Pérez Z, Geraci S, Friedemann J, Gretz N, Neiger R. A pilot study to assess the feasibility of transcutaneous glomerular filtration rate measurement using fluorescence-labelled sinistrin in dogs and cats. PLoS One 9: e111734, 2014. doi: 10.1371/journal.pone.0111734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tujjar O, Mineo G, Dell’Anna A, Poyatos-Robles B, Donadello K, Scolletta S, Vincent JL, Taccone FS. Acute kidney injury after cardiac arrest. Crit Care 19: 169, 2015. doi: 10.1186/s13054-015-0900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vandenberghe W, Gevaert S, Kellum JA, Bagshaw SM, Peperstraete H, Herck I, Decruyenaere J, Hoste EA. Acute kidney injury in cardiorenal syndrome Type 1 patients: a systematic review and meta-analysis. Cardiorenal Med 6: 116–128, 2016. doi: 10.1159/000442300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson DM, Turner DR, Cameron JS, Ogg CS, Brown CB, Chantler C. Value of renal biopsy in acute intrinsic renal failure. BMJ 2: 459–461, 1976. doi: 10.1136/bmj.2.6033.459. [DOI] [PMC free article] [PubMed] [Google Scholar]