Fig. 2.
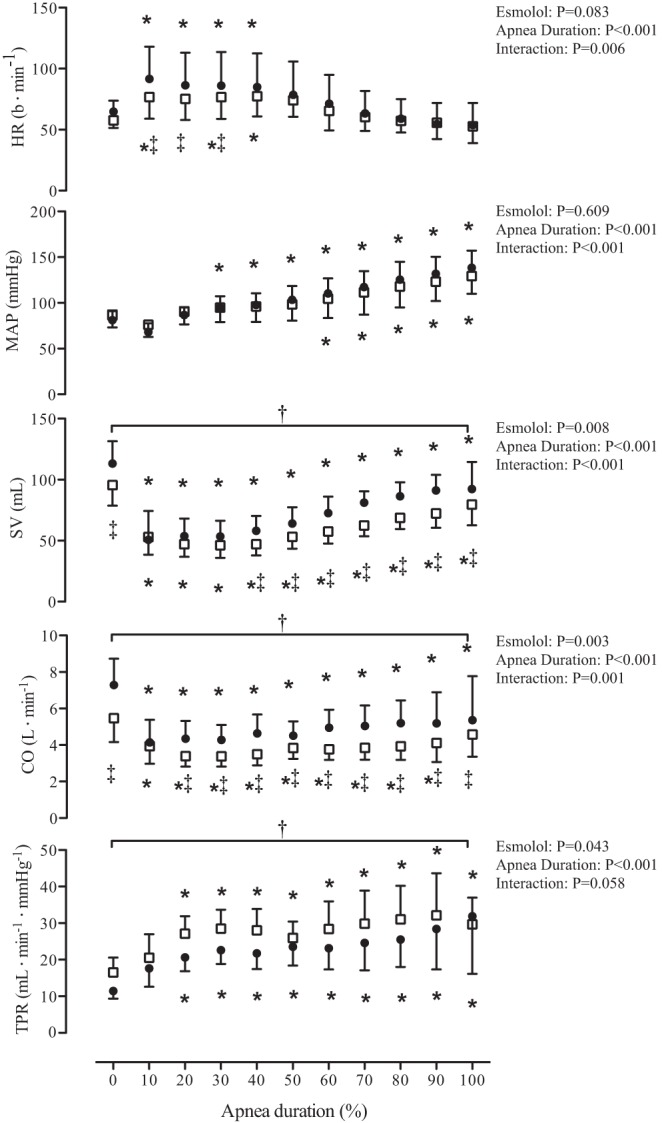
The hemodynamic response to maximal apnea during placebo and esmolol infusion. Data are normalized within subjects to represent 10% increments in apnea duration. ●, Data from the placebo trial; □, data from the esmolol trial. Data were analyzed using a 2-way repeated-measures ANOVA with the factors of apnea duration and esmolol treatment. *Significant difference from baseline P < 0.05; †main effect of treatment, P < 0.05; ‡interaction between apnea time and treatment. HR, heart rate; MAP, mean arterial pressure; SV, stroke volume; CO, cardiac output; TPR, total peripheral resistance.
