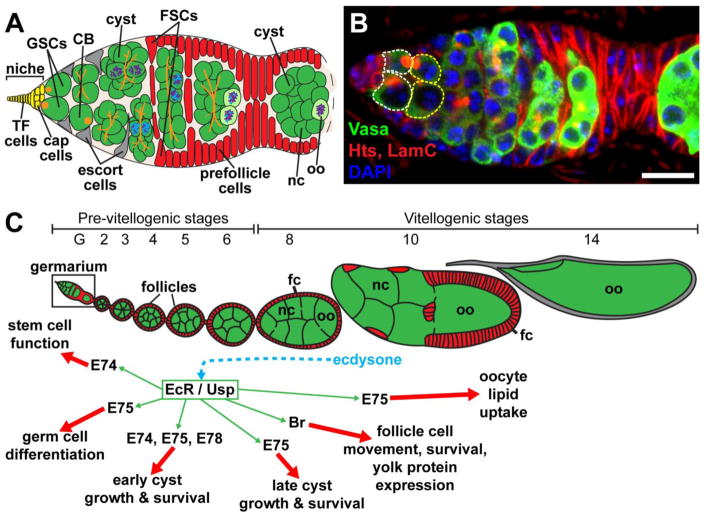
Figure 1. The steroid hormone ecdysone controls multiple steps of Drosophila oogenesis.
Female germline stem cells (GSCs) reside in a germarium (A, B) at the anterior tip of each of the 14–16 ovarioles (C) that compose the Drosophila ovary. (A) GSCs are anchored to adjacent cap cells that, along with terminal filament (TF) cells, send signals to maintain GSCs in a self-renewing fate. GSCs divide to form daughter cells (cystoblasts, CB), which divide four additional times to form 16-cell germline cysts composed of nurse cells (nc) and an oocyte (oo). Escort cells (gray) signal to germ cells to promote differentiation. Follicle stem cells (FSCs) divide to form prefollicle cells, which surround the 16-cell germline cyst, giving rise to a follicle that leaves the germarium. Prefollicle cells give rise to a variety of specialized follicle cells (fc; red) that form an epithelial monolayer around each cyst. (B) Confocal micrograph of a germarium immunolabeled with anti-Vasa (green; labels all germ cells), anti-Hts (red; labels early germline-specific organelles called fusomes and follicle cell membranes), anti-Lamin C (red; labels the nuclear envelope of cap cells), and DAPI (blue; labels all nuclei). Dashed lines outline GSCs (white) and cystoblasts (yellow). Scale bar, 10 μm. (C) Summary of ecdysone-regulated processes in the ovary. Ecdysone is produced by older follicles, and stimulates the EcR/Usp complex expressed throughout the ovary. Activation of the complex results in a variety of cellular responses mediated by distinct ecdysone-responsive transcription factors, such as E74, E75, E78, and Br.