Table 2.
Substrate scope for the α-carboxylation of amines with CO2.
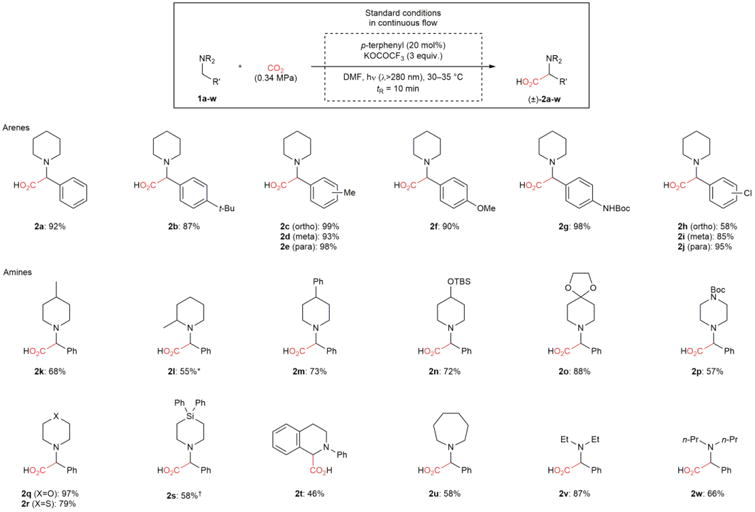
|
Various amino acids are synthesized in high yields and excellent regioselectivities (>20:1) with a short residence time. This protocol utilizes readily available starting materials and a commercially available, inexpensive organic catalyst. Products were isolated as trifluoroacetate salts on 0.7 mmol scale. (2s and 2t were isolated as free amines)
1.3:1 diastereomeric ratio was observed by GC analysis.
Reaction was carried out under atmospheric pressure of CO2 (1.1 equiv CO2) with tR = 5 min. Boc, tert-butyloxycarbonyl; TBS, tert-butyldimethylsilyl.
